Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
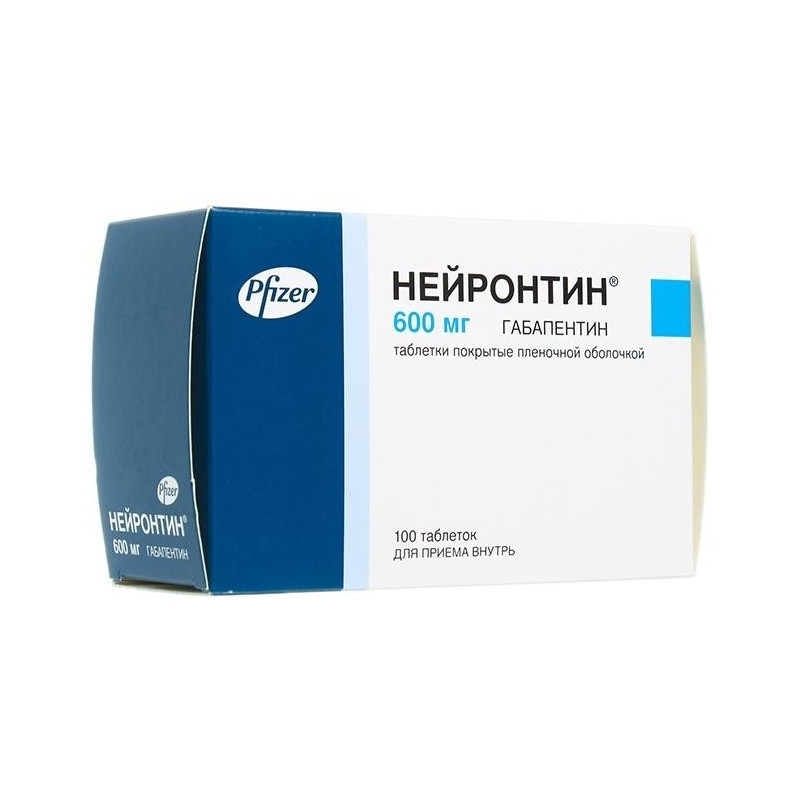
ALERT DETAILS A prescription that was recently filled for GABAPENTIN 600 MG TABLET may be part ofa recall or a safety alert. Patients may have the right to get an updated fill if the current one is affected. One needs a closer look for what sounds to be a slightly different reason than usual. The FDA has reported out that, Sun Pharmaceutical Industries, Inc. initiated a recall for 13,728 bottles of gabapentin, including 12,876 bottles containing 300-milligram dosage and 852 bottles containing 400 milligrams per dose. This recall is due to a potential mix-up of Metformin ER Tablets in a bottle of Gabapentin Tablets, USP 600mg. The ongoing investigation revealed that the issue is limited to the above lot and no other lots were impacted. The most recent Recall Enforcement Report that covers this product was initiated on July 31st, 2024 and classified as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets This recall is currently terminated, and the associated recall number is recall number is D-0634-2024. It pertains to Gabapentin identified by Usage, warnings, side effects, and community information for the prescription drug Gabapentin The Board of Pharmacy has received notice of the following product recall. The Board strongly encourages pharmacies to immediately review their quality assurance and recall policies and procedures to determine if any corrective action is required. The Harvard Drug Group, LLC d/b/a Major Pharmaceuticals and Rugby Laboratories has initiated a recall for Gabapentin Tablets, 600 mg. This recall Drug Recall Enforcement Report Class II voluntary initiated by Granules Pharmaceuticals Inc., originally initiated on 07-31-2024 for the product Gabapentin Tablets, USP, 600 mg, 500-count bottles, Rx only, Manufactured by: Granules India Limited Hyderabad-500 081, India, Manufactured for: Granules Pharmaceuticals Inc., Chantilly, VA NDC 70010-227-05 The product was recalled due to presence of Description: Gabapentin Tablets 600mg, 500-count bottles, Rx Only, Manufactured by: Glenmark Pharmaceuticals Limited, Pithampur, Madhya Pradesh 454775, India, Manufactured for: Glenmark Pharmaceuticals Inc. USA, Mahwah, NJ 07430. The most recent Recall Enforcement Report that covers this product was initiated on July 31st, 2024 and classified as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets This recall is currently completed, and the associated recall number is recall number is D-0634-2024. It pertains to Gabapentin identified by in a shared facilityw ith E ze tim ibe tab lets IOm g and E ze tim ibe / S im vastatin tab lets 10m g /IOm g , I0 O u to fan abundanceo fcau tion , the recall o f the abovem en tioned drug p roduc t batches, m anu factu red 100 'sC oun t (B a tch# 17231956). The recall affects gabapentin tablets, 600 mg, packaged in 100-tablet cartons (10 blister packs containing 10 tablets each, NDC 0904-6823-61), from lot T04468 (Exp 10/24). The cartons were distributed by Aurobindo Pharma USA, East Windsor, New Jersey, and Major Pharmaceuticals, Livonia, Michigan, throughout the United States. Description: Gabapentin Tablets, USP 600 mg, 500 tablets per bottle, RX Only, Manufactured by ScieGen Pharmaceuticals Inc., Hauppauge, NY 11788, NDC: 50228-177-05. Glenmark Pharmaceuticals recalls nearly 40 lots of generic medications over manufacturing issues impacting treatments for common health conditions. Drug Recall Enforcement Report Class III voluntary initiated by The Harvard Drug Group, originally initiated on 04-24-2023 for the product Gabapentin Tablets, USP 600 mg, packaged in Cartons of 100 tablets (10 tablets per blister pack x 10), Rx Only, Distributed by: Aurobindo Pharma USA, Inc. East Windsor, NJ 08520 Distributed by: Major Pharmaceuticals 17177 N Laurel Park Dr., Suite 233 Recall Enforment Report D-0354-2023 Recall Details Drug Recall Enforcement Report Class III voluntary initiated by Sciegen Pharmaceuticals Inc, originally initiated on 02-17-2023 for the product Gabapentin Tablets, USP 600 mg, 500 tablets per bottle, RX Only, Manufactured by ScieGen Pharmaceuticals Inc., Hauppauge, NY 11788, NDC: 50228-177-05. The Drug Recall Report is for prescription drugs that are have recently been issued recalls by the U.S. Food and Drug Administration (FDA). Gabapentin tablets 600 mg, 500-count bottles Lacosamide tablets 200 mg, 60-count bottle Frovatriptan succinate tablets 2.5 mg, 9-count bottle Rufinamide tablets 200 mg, 120-count bottle The most recent Recall Enforcement Report that covers this product was initiated on February 17th, 2023 and classified as a Class III recall due to presence of foreign tablets/capsules: pharmacist reported presence of some gabapentin tablets 800 mg comingled in gabapentin 600 mg 500 count bottles. This recall is currently terminated, and the associated recall number is recall number is D-0354 Description: Gabapentin Tablets, USP 600 mg, packaged in Cartons of 100 tablets (10 tablets per blister pack x 10), Rx Only, Distributed by: Aurobindo Pharma USA, Inc. East Windsor, NJ 08520 Distributed by: Major Pharmaceuticals 17177 N Laurel Park Dr., Suite 233 Livonia, MI 48152 USA, NDC 0904-6823-61 A website for the State of California, Department of Consumer Affairs, Board of Pharmacy
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |