Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
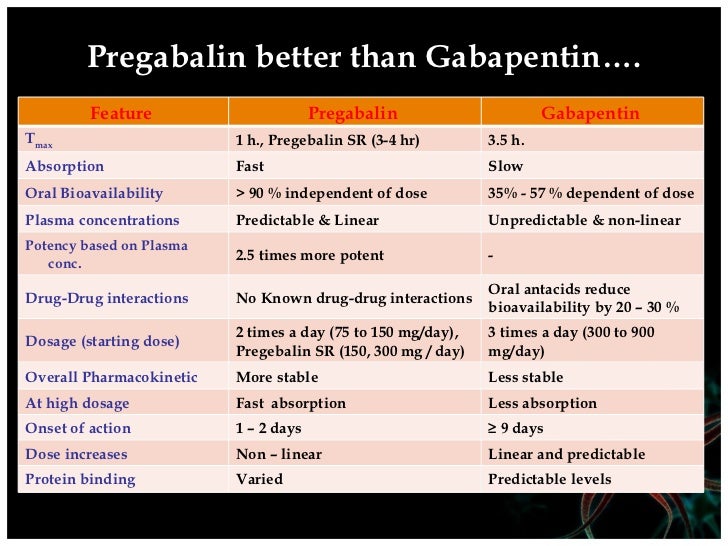
Pregabalin is a controlled drug in the US, gabapentin is controlled in some states and both will become controlled drugs in Great Britain in April 2019. Both Health Canada and the UK government have warned that gabapentin can cause severe respiratory depression, even without concomitant opioids. 6, 7, 8 Diversion of a drug is the illegal transfer of a prescription drug from medical sources or a patient for whom it was prescribed to others or the illicit black market. These drugs may end up on the streets. According to the DEA, gabapentin use is associated with sedative and/or psychedelic effects, similar to pregabalin. Both Lyrica and gabapentin are used as anti-epileptic medications and to treat nerve pain. But there are several differences between them. The main differences between Lyrica and gabapentin are: Lyrica is a brand name for pregabalin. Gabapentin is a generic name - brands of gabapentin include Neurontin, Gralise, and Horizant. Gabapentin is not the same as pregabalin, even though they both Gabapentin and pregabalin are similar drugs but differ in several distinct ways. The main differences are their indications—specific uses that the Food and Drug Administration (FDA) has approved them to treat—and their dosages. Gabapentin and pregabalin are FDA-approved to treat some of the same The landscape of prescription medications can be quite perplexing, especially when it comes to understanding why certain drugs are classified as controlled substances while others are not. A prime example of this is the difference between pregabalin and gabapentin. Both medications are used to treat similar conditions, primarily neuropathic pain and seizures, yet they are regulated very From 1st April 2019, amendments to the Misuse of Drugs Regulations 2001 and the Safe Custody Regulations 1973 come into force which mean that pregabalin and gabapentin will be reclassified as Schedule 3 Controlled Drugs (CDs). Briefing Note From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Pregabalin and gabapentin to be reclassified as Class C controlled substances Following a public consultation and advice from the Advisory Council on the Misuse of Drugs, gabapentinoids are to be reclassified following concerns over misuse. Re: Handling of gabapentin and pregabalin as Schedule 3 Controlled Drugs in health and justice commissioned services The purpose of this letter is to inform NHS England commissioned health and justice services and key stakeholders about the expectations for the handling of gabapentin and pregabalin as Schedule (Sch) 3 Controlled Drugs (CDs) from 1 April 2019. Both pregabalin and gabapentin from 1st April 2019 have been classified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971 in the UK. On the other hand, in the US, pregabalin is a Schedule 5 controlled substance while gabapentin is a controlled substance only in some States. Pregabalin (Lyrica) and gabapentin (Neurontin) are both approved to treat nerve pain. How are they different, and which one is preferred? Compare both meds here. The ACMD recommended that gabapentin and pregabalin be controlled as Class C drugs under the 1971 Act, and placed in Schedule 3 to the 2001 Regulations. For more information about these compounds From 1 April 2019, gabapentin and pregabalin have been reclassified as controlled drugs, leading to changes in how they are prescribed. All you need to know about: Pregabalin and gabapentin reclassification Here we explain the prescribing and dispensing changes that will need to take place when pregabalin and gabapentin become Schedule 3 Controlled Drugs. As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3 Gabapentin and pregabalin – controlled drugs demystified Gabapentin and pregabalin were reclassified as controlled drugs (CDs) back in April 2019, yet many health and social care providers still seem unsure how to treat them. Should gabapentin and pregabalin become approved additions to the existing list of controlled drugs available to physiotherapist and/or podiatrist independent prescribers, then such prescribers will again be able to prescribe them. NHS England and the professional bodies will update the professions if these changes are agreed. From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin Compare Gabapentin vs Pregabalin head-to-head with other drugs for uses, ratings, cost, side effects and interactions.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |