Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
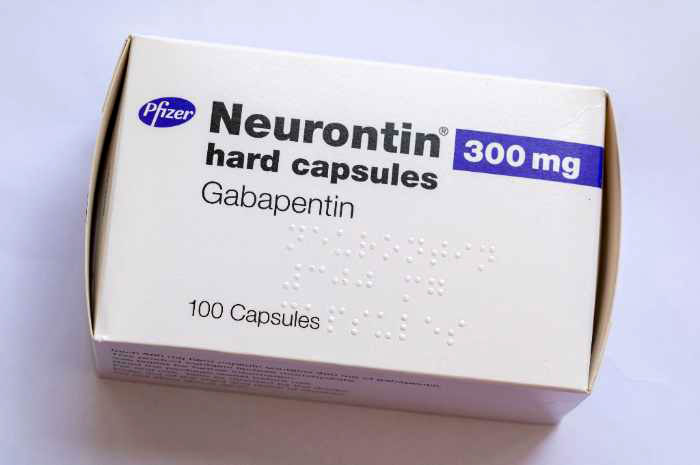
The manufacturers of the drug Neurontin reached a $325 million class action lawsuit settlement Wednesday over allegations they fraudulently marketed the prescription drug, which is used to treat seizures, restless leg syndrome, and pain caused by shingles. If approved, third-party payers of the drug will be eligible to claim a cash award from the Neurontin class action settlement, announced Explore the potential for seeking compensation in gabapentin-related memory loss cases, including legal grounds, evidence, and filing deadlines. The Neurontin class action settlement reached in April will apply to consumers in the United States that purchased Neurontin from Pfizer at any time during the period of December 11, 2002 through August 31, 2008, and who have purchased generic gabapentin. I have been taking Gabapentin for over 10 years, and it has really destroyed my teeth. I have Interstitial Cystitis and Fibromyalgia, and Gabapentin has helped me a great deal, so I need to keep taking it. I would definitely be interested in a Class Action lawsuit. If I could get my dental work done through this lawsuit I would be satisfied. The gabapentin class action lawsuit was filed in 2022 against three pharmaceutical companies – Teva, Pfizer, and Greenstone – accusing them of misrepresenting the drug’s risks and overstating its benefits. Pfizer Inc. has agreed to pay $325 million to resolve claims it defrauded insurers and other healthcare benefit providers by marketing Neurontin for unapproved uses. In the decade old class action lawsuit, TBS client, ASEA/AFSCME Local 52 Health Benefits Trust (ASEA), alleged that Pfizer and Warner-Lambert Co. sold Neurontin in an effort to boost profits How To Join Gabapentin Lawsuit? In this insightful video, we delve into the process of joining a Gabapentin lawsuit. If you've been prescribed Gabapentin and believe you've suffered harm from its Serving as co-lead counsel, Garwin Gerstein & Fisher LLP achieved a $190 million recovery for the Direct Purchaser Class. Direct purchasers claimed that Pfizer violated federal antitrust laws by illegally delaying the entry of generic versions of the prescription drug Neurontin. The active ingredient in Neurontin is gabapentin anhydrous. The lawsuit claimed that Pfizer delayed competition from Class action lawsuit settlements & rebates you can claim — many with no proof of purchase required! Check us daily for cash you can claim. Consumer Action maintains this listing of notable class actions so that interested consumers can learn more, join a pending action or make a claim. You can sort the listing three ways—actions or settlements that are (1) open to claims, (2) pending or (3) closed—or use the calendar to search for upcoming claims deadlines. A $190 million settlement has been reached in New York in a consumer fraud class action lawsuit pending against Pfizer which alleges the pharma giant engaged in tactics to delay market entry of generic versions of its epilepsy drug Neurontin. The lawsuit was filed by purchasers of Neurontin in 2002, claiming Pfizer The Neurontin settlement comes in a class action lawsuit brought by buyers of the drug, who claimed that Pfizer tried to illegally maintain exclusivity on the drug and keep prices artificially high. ClassAction.org’s class action lawsuit database is updated each day with many of the latest class action case filings from courts nationwide. Neurontin Lawsuit | 2025 Latest Updates The prescription pain reliever Neurontin (generic gabapentin) has recently been linked to an increased risk for Stevens-Johnson syndrome (SJS), a severe skin disorder in which the top layer of the skin dies, followed by a painful rash that spreads and blisters. Visit OpenClassActions.com to get the latest news on class action lawsuits, class action settlements, and rebates! In recent years, the manufacturers of Gabapentin have faced legal challenges as a result of these issues. One notable lawsuit emerged when the manufacturers of Neurontin reached a $325 million class action lawsuit settlement over allegations of fraudulent marketing practices. Neurontin Lawsuits Neurontin (generic name: gabapentin) was FDA-approved in 1993 for use as an anticonvulsant for people suffering from partial seizures associated with epilepsy. Consumers are filing lawsuits against prescription drug and medical device companies after facing serious injuries. Find out if you qualify for a lawsuit. Los Angeles, CA: A $325 million preliminary settlement has been reached by Pfizer Inc and Warner-Lambert Co. LLC and plaintiffs who filed a consumer fraud class action lawsuit over the marketing If you or a family member has had any of the aforementioned Neurontin adverse effects, especially if it was administered for an unapproved purpose, you should contact an experienced class action attorney to talk about your case and learn more about your options for compensation for a Neurontin injury.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |