Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 | 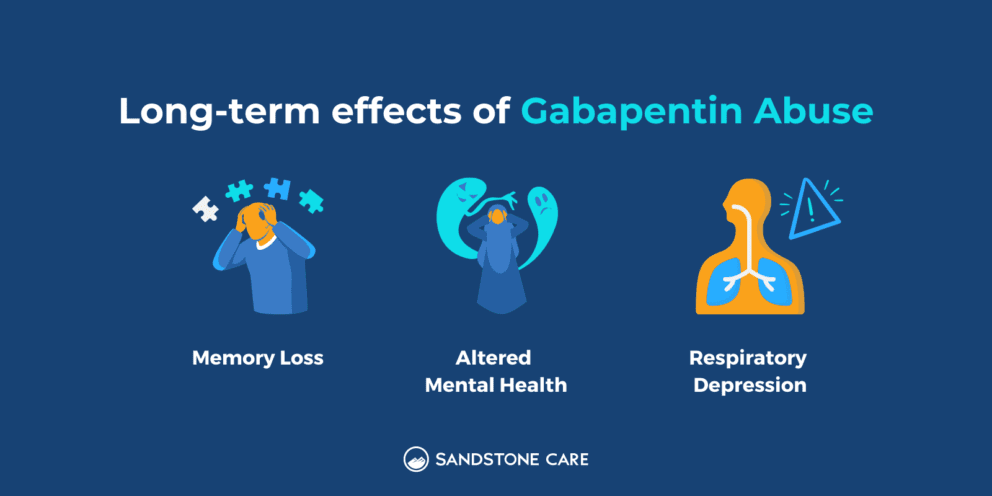 |
Having originally been developed as an anti-seizure medication, gabapentinoids include gabapentin and pregabalin, which are now prescribed primarily for neuropathic pain, seizures and anxiety, but also for fibromyalgia, restless legs syndrome and complications of MS (Chan et al, 2023). First introduced in the UK and the US in 1993, the number of doses of pregabalin and gabapentin taken daily The UK government reclassified gabapentin and pregabalin as ‘controlled drugs’ from April 2019. This study aimed to describe the trends in gabapentinoid prescribing before and immediately after reclassification, in the UK Clinical Practice Research Datalink, an electronic primary care health record broadly representative of the UK. From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. Their reclassification follows a Government consultation and recommendations from the Advisory Council on the Misuse of Drugs that additional safeguards As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3 The UK government announced last week that the medicines pregabalin and gabapentin will be reclassified as class C under the Misuse of Drugs Act 1971. The change will take place in April 2019. Class C is the third in the government’s three-tier system for categorising controlled substances. Important changes affecting Gabapentin and Pregabalin prescriptions. Gabapentin and Pregabalin are now classified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. The most commonly encountered drugs currently controlled under the misuse of drugs legislation. The government’s reclassification was expected to reduce prescribing of gabapentinoids, but Elisabeth Mahase finds that a lack of support for GPs and patients is limiting its impact In October 2018, the UK government announced that it would be reclassifying gabapentin and pregabalin1—known collectively as gabapentinoids—after experts pointed out the rising numbers of deaths linked to the All you need to know about: Pregabalin and gabapentin reclassification Here we explain the prescribing and dispensing changes that will need to take place when pregabalin and gabapentin become Schedule 3 Controlled Drugs. From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin Gabapentin is licensed for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and postherpetic neuralgia in adults [ABPI, 2020a]. However, the National Institute for Health and Care Excellence (NICE) recommends gabapentin as a first-line treatment option for adults with all neuropathic pain (except trigeminal neuralgia) [NICE, 2019a]. Gabapentin is recommended for use in focal seizures and neuropathic pain. [7][10] Gabapentin is prescribed off-label in the US and the UK, [22][23] for example, for the treatment of non-neuropathic pain, [22] anxiety disorders, sleep problems and bipolar disorder. [24] In recent years, gabapentin has seen increased use, particularly in the elderly. [25] There is concern regarding gabapentin's Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. The UK government is to reclassify the prescription drug pregabalin as a class C controlled substance, after experts issued safety warnings following an increase in deaths linked to its use. A Home Office consultation, which also proposes reclassifying gabapentin, has been launched in response to growing pressure for the drug to be reclassified to tackle misuse and addiction. Last year the Gabapentin and pregabalin – controlled drugs demystified Gabapentin and pregabalin were reclassified as controlled drugs (CDs) back in April 2019, yet many health and social care providers still seem unsure how to treat them. Are they controlled drugs? 3. Gabapentin and pregabalin – Class C Gabapentin and pregabalin are anticonvulsant drugs. These have an established role in the management of a number of disabling long-term conditions. Briefing Note From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |