Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
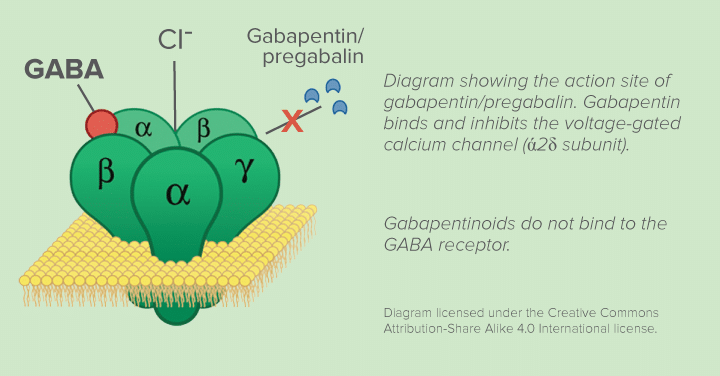
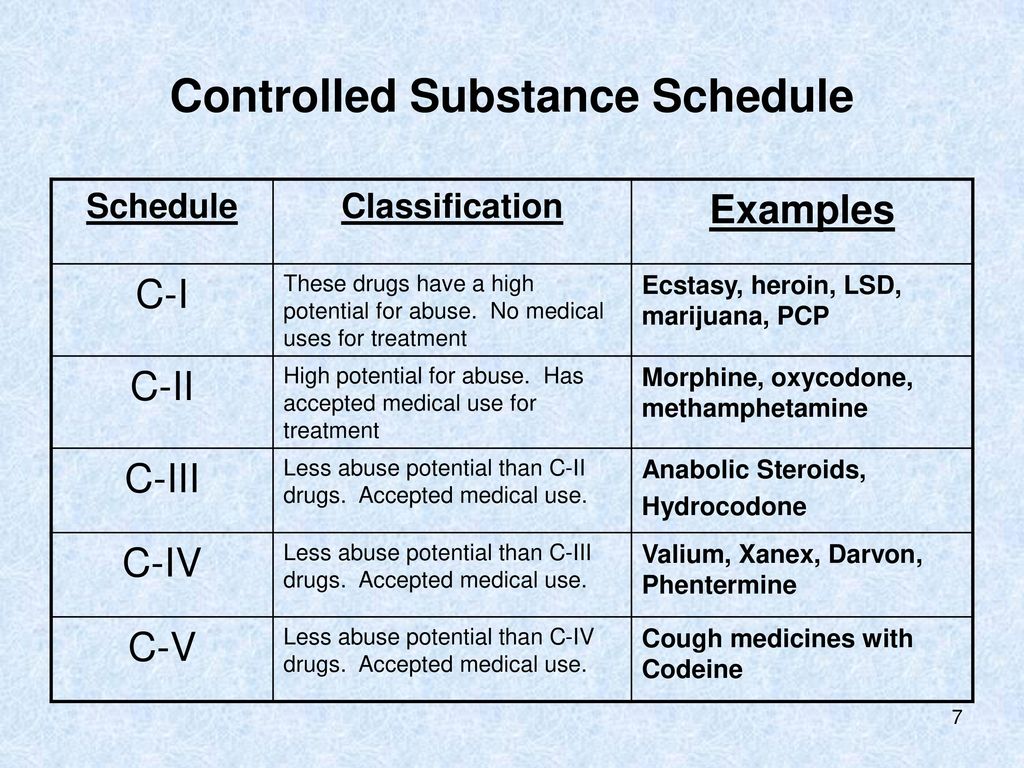
We compared two interventions including (1) class V scheduling of gabapentin and (2) gabapentin inclusion in the state PDMP using a control group of similar states. The Schedule V change for gabapentin was implemented in three states including Kentucky, Tennessee, and West Virginia (Table 1). Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin is not a narcotic or federally controlled substance, but it is classified as a Schedule V drug in certain states due to its potential for abuse and diversion. Learn which states control gabapentin, why it is regulated, and how it can interact with opioids and other drugs. This annual publication of the Texas Schedules of Controlled Substances was signed by Jennifer A. Shuford, M.D., M.P.H., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk of overdose death when used in combination with them. The US has seen substantial increases in If gabapentin is, or becomes, a controlled substance in your state, it does not necessarily mean it will be more difficult to obtain. Rather, it is a safety measure to assure we are using medications appropriately. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Only practitioners with a Utah Controlled Substance license and a DEA registration may issue prescriptions for Gabapentin or order the direct administration or dispensing of Gabapentin to a patient. This bill amends Montana's drug scheduling law to add gabapentin to Schedule V controlled substances, which is a category of drugs with lower potential for abuse. Currently, Schedule V includes certain narcotic drugs with limited quantities of non-narcotic ingredients, stimulants, depressants, anticonvulsant substances, and antimigraine medications. Specifically, the bill inserts gabapentin RX DRUG SCHEDULING & MONITORING Prescription Drug Monitoring Programs (PDMPs) are electronic databases that collect information on the dispensing and prescribing of drugs within jurisdictions. PDMPs aim to assist patients in their quality of care by allowing prescribers and dispensers access to the patient’s controlled substance prescription medication history. This access to individual Key takeaways: Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule In accordance with a new state law, the anticonvulsant and nerve pain medication gabapentin will soon be added to the list of drugs tracked through the state’s prescription drug management program (PDMP), the NC Controlled Substances Reporting System (NC CSRS). While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73 (b) adds it to the Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. “Using a data-driven approach, we identified Gabapentin as an emerging threat in our state and took necessary action to protect Michigan residents,” said LARA Director Orlene Hawks. In Pennsylvania, the regulation of Gabapentin has become an important issue as officials attempt to control its prescribed and non-medical usage. In 2019, Pennsylvania passed new regulations that categorized Gabapentin as a Schedule V controlled substance. This change was made due to concerns about the drug’s misuse potential. Gabapentin is chemically known as 2-[1-(aminomethyl) cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. However, some states have implemented their laws to reclassify gabapentin as a Schedule V controlled substance. In states that label gabapentin as a controlled substance, there may be regulations mandating specific requirements for prescriptions, as well as limits on the quantity prescribed or refills available. We would like to show you a description here but the site won’t allow us.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |