Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
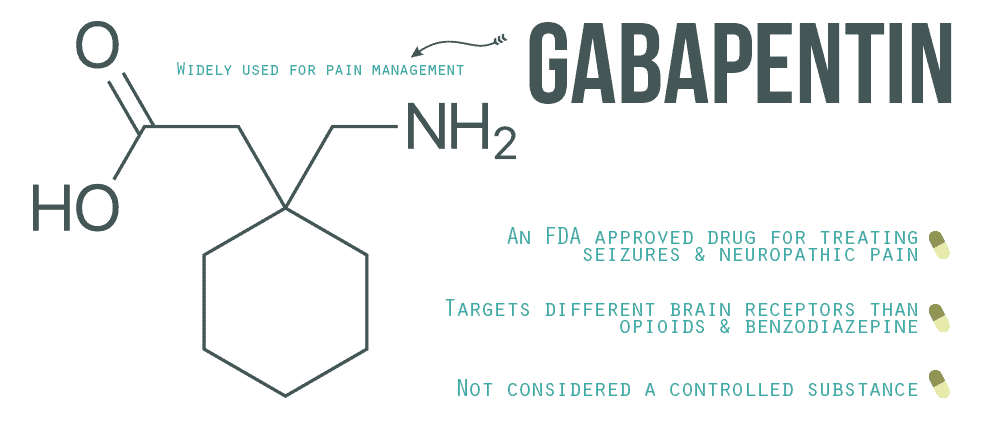
Gabapentin is chemically known as 2-[1-(aminomethyl) cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedati We would like to show you a description here but the site won’t allow us. Gabapentin isn’t classified as a controlled substance under federal law in the United States. But it is classified as a controlled substance in some states. The number of states placing gabapentin on the controlled substance list or in their required monitoring program is growing and three more states are debating whether to add gabapentin as a controlled substance or to their mandated reporting programs (DE, NY, and WI). Will Gabapentin prescription histories be available in the Controlled Substance Reporting System? • Yes, Gabapentin prescription histories will be available to practitioners with a Controlled Substances Reporting System (CSRS) registration. Gabapentin is used to treat many conditions, including seizures and pain conditions. Gabapentin is not a controlled substance on a federal level but is controlled in some states, which limits the number of prescription refills and how it is reported. Gabapentin can be dangerous when used in combination with other substances, particularly opioids. However, some states have implemented their laws to reclassify gabapentin as a Schedule V controlled substance. In states that label gabapentin as a controlled substance, there may be regulations mandating specific requirements for prescriptions, as well as limits on the quantity prescribed or refills available. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin – or Neurontin – is a medication commonly used to treat nerve pain and seizures. However, the drug can have potentially harmful effects when combined with other opioids. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. Gabapentin is a prescription medication that falls into a class of drugs known as anticonvulsants, sometimes called anti-epileptic drugs. It works by reducing specific nerve signals sent to the brain, helping you cope with chronic pain and improve alertness. See Epilepsy. MHRA/CHM advice: Gabapentin (Neurontin ®) and risk of abuse and dependence: new scheduling requirements from 1 April (April 2019) Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Gabapentin is not a federally-controlled drug substance and does not contain an opioid (narcotic) medication. However, gabapentin misuse and abuse has been reported, and it may be restricted in some states through their state drug-monitoring program. Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk of overdose death when used in combination with them. The US has seen substantial increases in Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial Gabapentin, initially developed for epilepsy, is now widely used for nerve pain and other off-label applications. Rising prescription rates have sparked discussions about whether it should be classified as a controlled substance due to concerns over misuse and dependency. Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |