Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
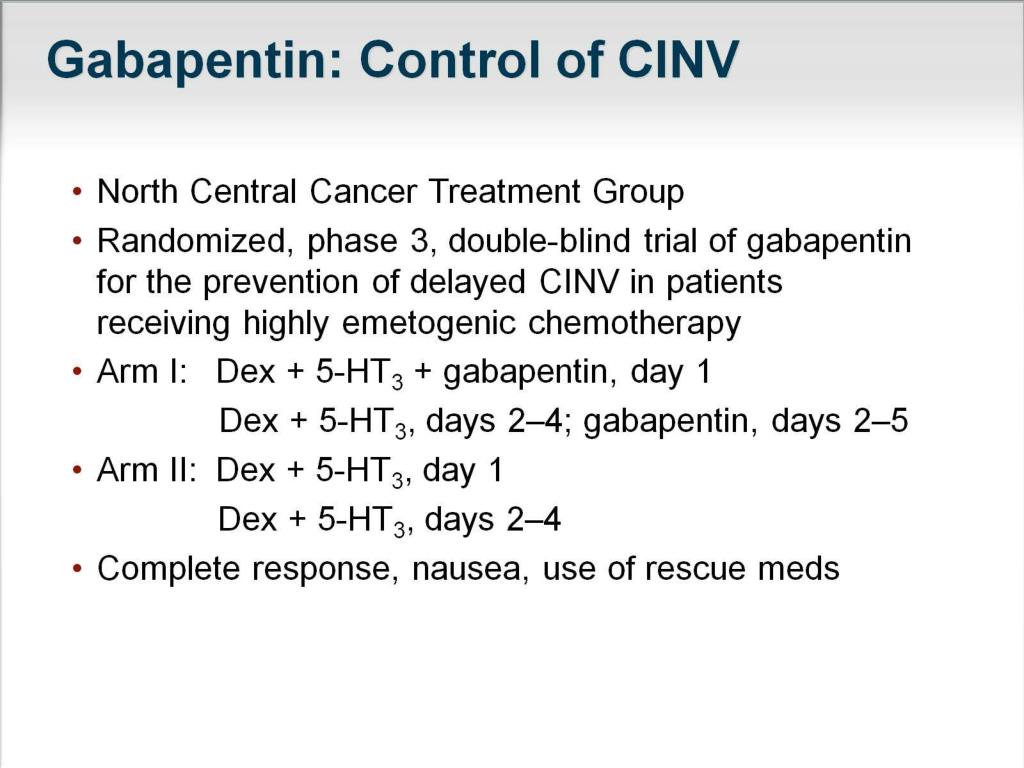
Gabapentin is prescribed frequently for many symptoms and often in an attempt to minimize the use of opioid pain medications. Read more.. See Epilepsy. MHRA/CHM advice: Gabapentin (Neurontin ®) and risk of abuse and dependence: new scheduling requirements from 1 April (April 2019) Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. There are five groups of controlled substances. Drug Info Is gabapentin a controlled substance? Gabapentin is not currently classified as a controlled substance. Here’s what to know about the refill laws. Gabapentin is a non-opioid pain medication for treating neuropathy and seizures caused by epilepsy. Although rates of gabapentin misuse are rising, the drug is not federally regulated as a controlled substance. Several states, however, have enacted laws in recent years to list gabapentin as a controlled substance. Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Due to concerns about the potential misuse of gabapentin, some states have classified the drug as a controlled substance. Other states have put stricter measures in place to monitor possible misuse of gabapentin. We would like to show you a description here but the site won’t allow us. Gabapentin is commonly used to treat some types of nerve pain but is classified as an anticonvulsant medicine, not as an opioid or painkiller. Gabapentin was first approved in 1993 and is used to treat: postherpetic neuralgia, a nerve pain caused by the shingles virus (herpes zoster), restless legs syndrome (RLS), a painful movement disorder in the legs partial seizures in adults and children Medication-assisted treatment (MAT): MAT is an evidence-based approach that combines medication with behavioral therapy to reduce cravings and withdrawal symptoms associated with substance abuse disorders. Currently, no medications are explicitly approved for treating gabapentin abuse and addiction. Gabapentin is used to treat many conditions, including seizures and pain conditions. Gabapentin is not a controlled substance on a federal level but is controlled in some states, which limits the number of prescription refills and how it is reported. Gabapentin can be dangerous when used in combination with other substances, particularly opioids. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. However, healthcare providers also prescribe the drug off-label to treat conditions such as fibromyalgia, anxiety, and bipolar disorder. There have been increasing reports of gabapentin misuse, either alone or with opioids to enhance their euphoric (“high”) effects. At the federal level, gabapentin is not a controlled substance. 9.1 Controlled Substance - Gabapentin tablet contains gabapentin, which is not a controlled substance. 9.2 Abuse - Abuse is the intentional, non-therapeutic use of a drug, even once, for its Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways to enhance patient safety when prescribing gabapentin. Source: National Library of Medicine National Center for Biotechnology Information
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |