Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
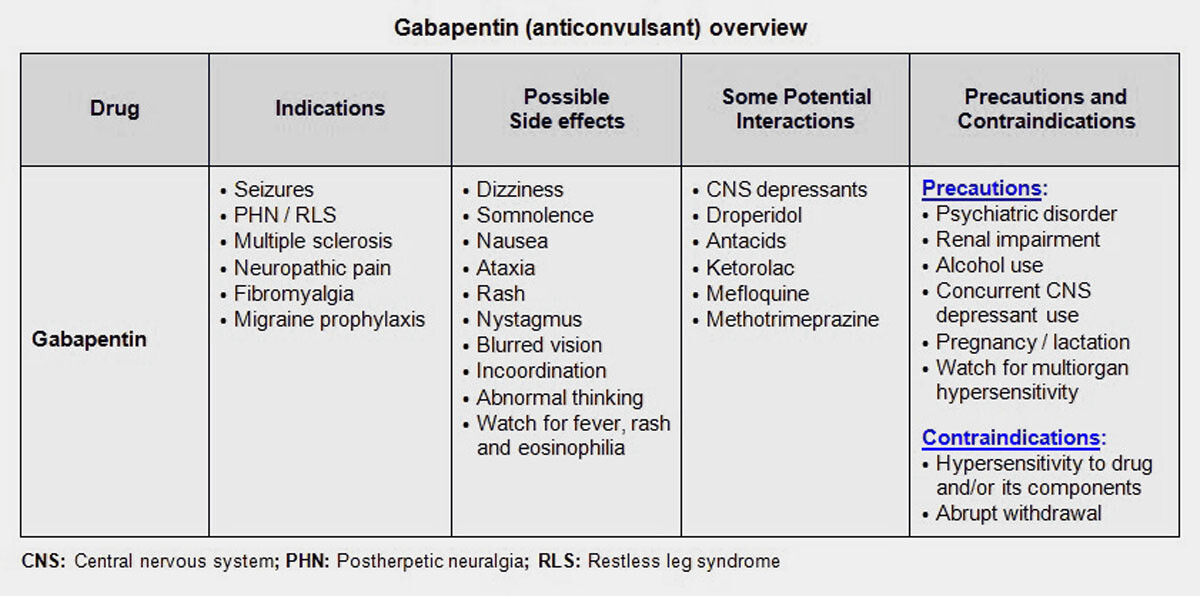
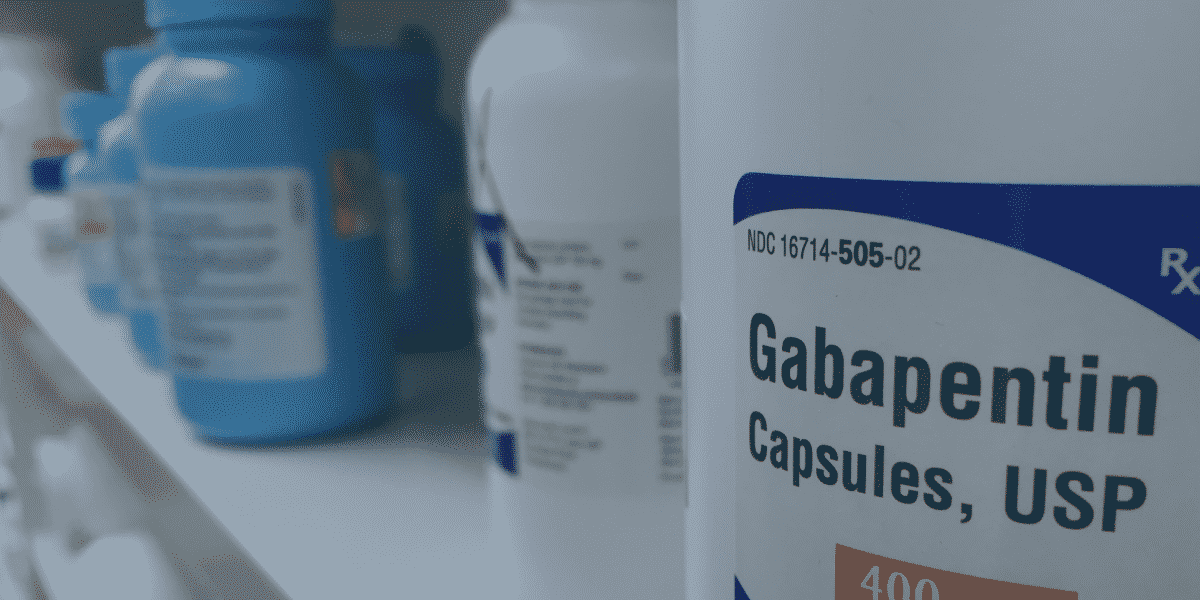
With mounting evidence of misuse and abuse of gabapentin use, certain states have implemented regulations or policies to limit or monitor the use of the drug, especially given its potential to enhance the effects of opioids. Some experts and nonprofit groups have called for national reclassification of gabapentin as a controlled substance. The information includes: the patient’s name, address, phone number, date of birth, sex, pharmacy and prescriber information, and specific prescription information including the drug name and dosage, when it was prescribed, and when it was dispensed. This is only for prescriptions that are classified as controlled substances Schedules II, III and IV and gabapentin. 37-2726. Filing prescriptions — Database. (1) All controlled substances and opioid antagonists as defined in section 54-1704, Idaho Code, dispensed for humans shall be filed with the division electronically in a format established by the division. The division may require the filing of other prescriptions by rule. The division shall establish the information to be submitted pursuant to the Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here. The Idaho Controlled Substance Registration (CSR) is required for practitioners who will prescribe, administer, order or store controlled substances. Per Idaho law, once the Idaho CSR is issued, an associated DEA must be obtained within 45 days. Once issued a primary Idaho CSR, any additional Idaho practice address requires a separate Idaho CSR and associated DEA only if the practitioner will 37-2707. Schedule II. (a) Schedule II shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Gabapentin, initially developed for epilepsy, is now widely used for nerve pain and other off-label applications. Rising prescription rates have sparked discussions about whether it should be classified as a controlled substance due to concerns over misuse and dependency. 37-2722. issuing, distributing, and dispensing of controlled substances. No person shall issue or dispense a prescription drug order for a controlled substance unless it is in compliance with applicable state and federal law and rules of the board. In addition to the penalties prescribed in article IV of the uniform controlled substances act, any person shall be guilty of a felony who prescribes, dispenses, supplies, sells, delivers, manufactures or possesses with the intent to prescribe, dispense, supply, sell, deliver or manufacture anabolic steroids or any other human growth hormone Due to concerns about the potential misuse of gabapentin, some states have classified the drug as a controlled substance. Other states have put stricter measures in place to monitor possible misuse of gabapentin. TITLE 37 FOOD, DRUGS, AND OIL CHAPTER 27 UNIFORM CONTROLLED SUBSTANCES ARTICLE II 37-2711. Schedule IV. (a) Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. (b) Narcotic drugs. Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. The number of states placing gabapentin on the controlled substance list or in their required monitoring program is growing and three more states are debating whether to add gabapentin as a controlled substance or to their mandated reporting programs (DE, NY, and WI). 2024 Idaho Code Title 37 - FOOD, DRUGS, AND OIL Chapter 27 - UNIFORM CONTROLLED SUBSTANCES Article III Section 37-2722 - ISSUING, DISTRIBUTING, AND DISPENSING OF CONTROLLED SUBSTANCES. TITLE 37 FOOD, DRUGS, AND OIL CHAPTER 27 UNIFORM CONTROLLED SUBSTANCES ARTICLE II 37-2713. Schedule V. (a) Schedule V shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. (b) Narcotic drugs. 2014 Idaho Statutes Title 37 - FOOD, DRUGS, AND OIL Chapter 27 - UNIFORM CONTROLLED SUBSTANCES Article III Section 37-2722 - PRESCRIPTIONS. Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial Gabapentin is used to treat many conditions, including seizures and pain conditions. Gabapentin is not a controlled substance on a federal level but is controlled in some states, which limits the number of prescription refills and how it is reported. Gabapentin can be dangerous when used in combination with other substances, particularly opioids. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. TITLE 37 FOOD, DRUGS, AND OIL CHAPTER 27 UNIFORM CONTROLLED SUBSTANCES Download Entire Chapter (PDF) ARTICLE I ARTICLE II ARTICLE III ARTICLE IV ARTICLE V ARTICLE VI
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |