Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
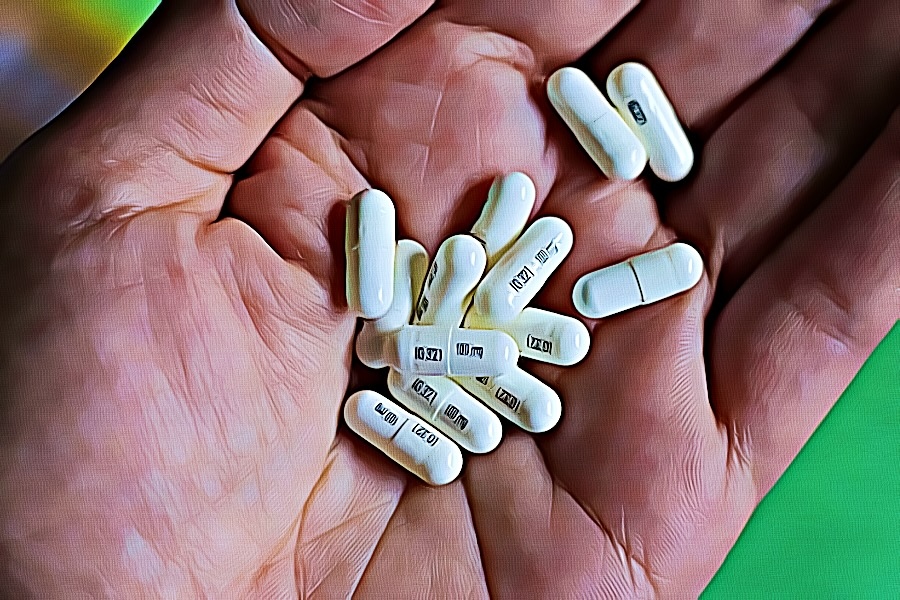
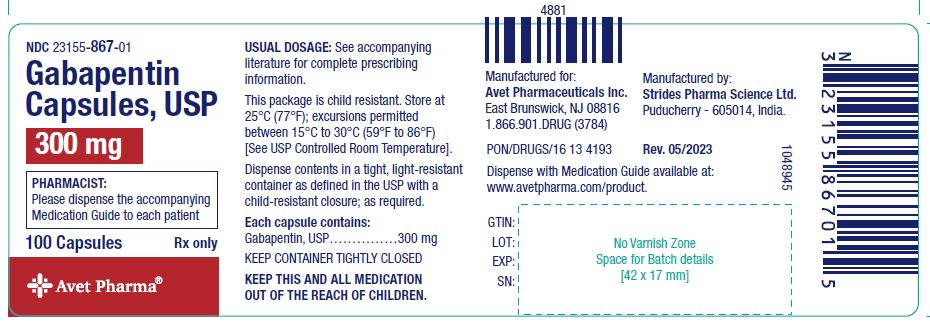
Pharmacies licensed and located in Minnesota must report to the MN PMP all schedule II-V controlled substance prescriptions, along with prescriptions for butalbital and gabapentin, when dispensed. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Pharmacies licensed and located in Minnesota must report to the MN PMP all schedule II-V controlled substance prescriptions, along with prescriptions for butalbital and gabapentin, which are dispensed. A controlled substance is a substance with a higher likelihood for addiction and dependence. Here, we’ll talk about the risks associated with gabapentin and why some states consider it a controlled substance. The pharmacy may accept cash payment for a noncovered controlled substance or gabapentin after the prescriber and member complete their sections of the Advance Member Notice of Noncovered Prescription (DHS-3641), and the prescriber also confirms all of the following: Gabapentin is not classified as a controlled substance on the federal level. However, there are growing concerns about the potential risks. Here’s what to know. We would like to show you a description here but the site won’t allow us. Dispensers (pharmacies) licensed by the Board of Pharmacy must report daily, all MN schedule II-V controlled substances, butalbital and gabapentin prescriptions to the MN PMP when dispensed in or into the State. Guidelines for the use of controlled substances United State CDC Guideline for Prescribing Opioids for Chronic Pain — March, 2016 FSMB Model Policy on the Use of Opioid Analgesics in the Treatment of Chronic Pain - July 2013 The Joint Statement of the Minnesota Boards of Medical Practice, Nursing, and Pharmacy on Pain Management - December 2015 Gabapentin isn’t classified as a controlled substance under federal law in the United States. But it is classified as a controlled substance in some states. The report must include specific recommendations for amending the controlled substance schedules contained in subdivisions 2 to 6, so that they conform with the controlled substance schedules maintained by the board in Minnesota Rules, parts 6800.4210 to 6800.4250, and with the federal schedules. Answer: The Minnesota Prescription Monitoring Program (PMP) is administered by the MN Board of Pharmacy (BOP) and collects prescription data on all schedules II-V controlled substances as well as butalbital and gabapentin regardless of how the prescription was paid for (cash, insurance, etc.). The MN BOP was given authority under Minnesota Statute M.S. § 152.126 to establish a program with For the purposes of this section, controlled substances includes butalbital and gabapentin. (d) "Dispense" or "dispensing" has the meaning given in section 151.01, subdivision 30. Dispensing does not include the direct administering of a controlled substance to a patient by a licensed health care professional. Controlled substance prescriptions as defined in M.S.152.126 (Schedules II-V, butalbital and gabapentin) dispensed at this pharmacy are reported to the Minnesota Prescription Monitoring Program as required by Minnesota Statutes Section 152.126 and may be used for program administration purposes. In Minnesota, gabapentin is not a scheduled controlled substance, but doctors are required to report prescriptions to a Prescription Drug Monitoring Program. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Required Reporting of Controlled Substances Dispensers (pharmacies) licensed by the MN Board of Pharmacy must report daily, all Minnesota schedule II-V controlled substances, butalbital and gabapentin prescriptions to the Minnesota Prescription Monitoring Program (MN PMP), when dispensed in or into the State. Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial Required Reporting of Controlled Substances Dispensers (pharmacies) licensed by the MN Board of Pharmacy must report daily, all Minnesota schedule II-V controlled substances, butalbital and gabapentin prescriptions to the Minnesota Prescription Monitoring Program (MN PMP), when dispensed in or into the State. Controlled Substance Insight Alerts The MN PMP provides Controlled Substance Insight Alerts (CSIAs) to prescribers and/or pharmacies regarding patients to which they have provided care and who appear to be receiving controlled substance medications from multiple prescribers and pharmacies.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |