Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
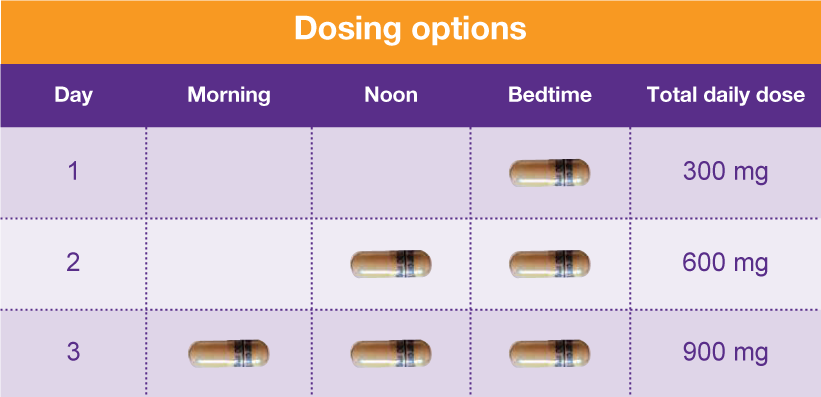 | 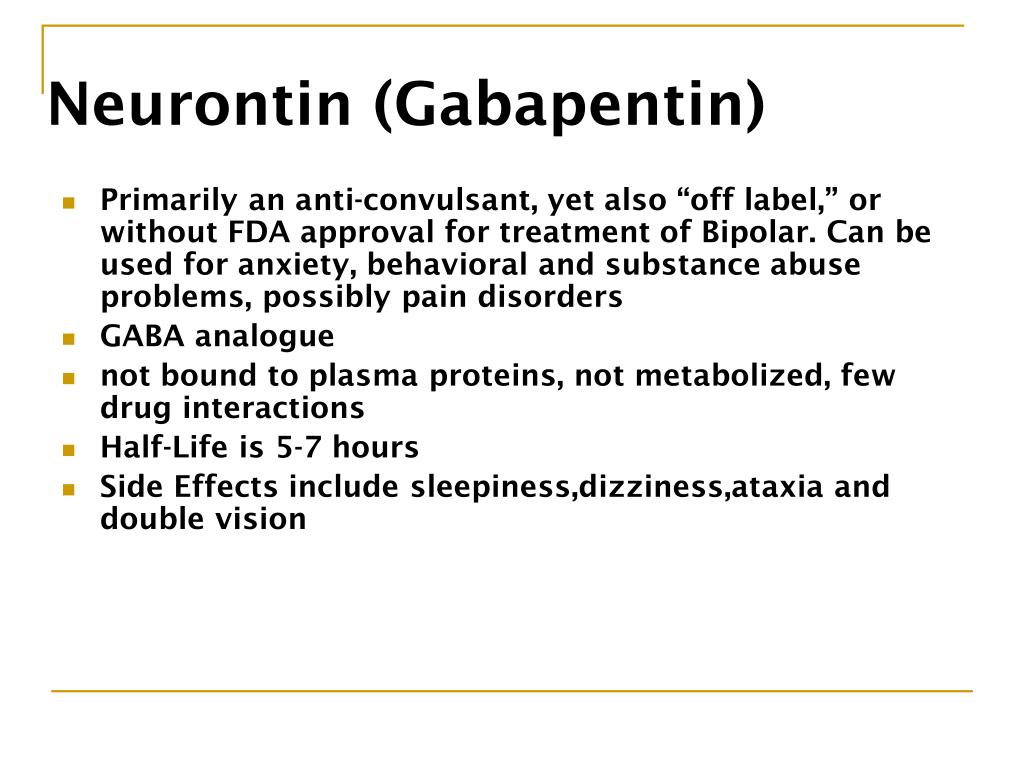 |
The Oregon Prescription Drug Monitoring Program (PDMP) is a tool to help healthcare providers and pharmacists provide patients better care in managing their prescriptions. Gabapentin is an antiepileptic drug prescribed for several neuropathic pain conditions. This study aimed to evaluate gabapentin (GAB) trough plasma concentration range and the applicability of therapeutic drug monitoring in patients with neuropathic pain. Therapeutic drug monitoring may be useful due to inter-individual variation in pharmacokinetics and dose-dependent bioavailability; specimens for measurements should be collected before the morning dose since the short half-life may affect the interpretation of the concentration. Given the distinctive characteristics of both epilepsy and antiepileptic drugs (AEDs), therapeutic drug monitoring (TDM) can make a significant contribution to the field of epilepsy. The measurement and interpretation of serum drug concentrations The antiepileptic drugs with the strongest justifications for drug monitoring are lamotrigine, oxcarbazepine, stiripentol, and zonisamide. Stiripentol and tiagabine are strongly protein bound and are candidates for free drug monitoring. The aim of the study was to investigate the use and pharmacokinetic variability of gabapentin in epilepsy and non-epilepsy indications and to further evaluate the use of TDM in patients with restless legs syndrome (RLS). Background/Objectives: Gabapentin has variable pharmacokinetics (PK), which contributes to difficulty in dosing and increased risk of adverse events. The objective of this study was to leverage gabapentin concentrations from therapeutic drug monitoring (TDM) to develop a population PK (popPK) model and characterize significant covariates that impact gabapentin PK. Methods: Data were Background/Objectives: Gabapentin has variable pharmacokinetics (PK), which contributes to difficulty in dosing and increased risk of adverse events. The objective of this study was to leverage gabapentin concentrations from therapeutic drug monitoring (TDM) to develop a population PK (popPK) model and characterize significant covariates that impact gabapentin PK. Methods: Data were Optimize drug therapy and monitor patient adherence. ||Separate serum or plasma from cells within 2 hours of collection. Transfer 1 mL serum or plasma to an ARUP standard transport tube. (Min: 0.2 mL) Plain red. Also acceptable: Dark green (sodium or lithium heparin), lavender (K2 or K3EDTA) or pink (K2EDTA). A prescription drug monitoring program (PDMP) is an electronic database that tracks controlled substance prescriptions. Information from PDMPs can help clinicians identify patients who may be at risk for overdose and provide potentially lifesaving information and interventions. Therapeutic drug monitoring may be useful due to inter-individual variation in pharmacokinetics and dose-dependent bioavailability; specimens for measurements should be collected before the morning dose since the short half-life may affect the interpretation of the concentration. The aim of this study was to develop a population PK model for gabapentin appropriate for monitoring patients with neuropathic pain and for individualizing their dose regimens. Therapeutic urine drug monitoring of gabapentin is important for ensuring compliance to treatment strategies. Urine or oral fluid are the specimens of choice for routine monitoring of patients taking prescription drugs. RX DRUG SCHEDULING & MONITORING Prescription Drug Monitoring Programs (PDMPs) are electronic databases that collect information on the dispensing and prescribing of drugs within jurisdictions. PDMPs aim to assist patients in their quality of care by allowing prescribers and dispensers access to the patient’s controlled substance prescription medication history. This access to individual Gabapentin is an anticonvulsant agent which requires standard drug monitoring protocols to avoid drug abuse and misuse of prescriptions. This activity outlines the indications, mechanisms of action, administration, significant adverse effects, contraindications, monitoring, and characteristics of gabapentin toxicity. Gabapentin is a structural analogue of GABA used in the treatment of the partial epilepsies of adult and child of more than 12 years, in monotherapy or in association with other anticonvulsant drugs. In association, gabapentin presents the advantage of not interfering with the other anticonvulsant d Gabapentin has been increasingly used in various indications in recent years. Despite variable pharmacokinetics, therapeutic drug monitoring (TDM) is scarcely described in other indications than epilepsy. Monitoring serum gabapentin concentrations Assessing compliance Adjusting dosage in patients View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |