Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
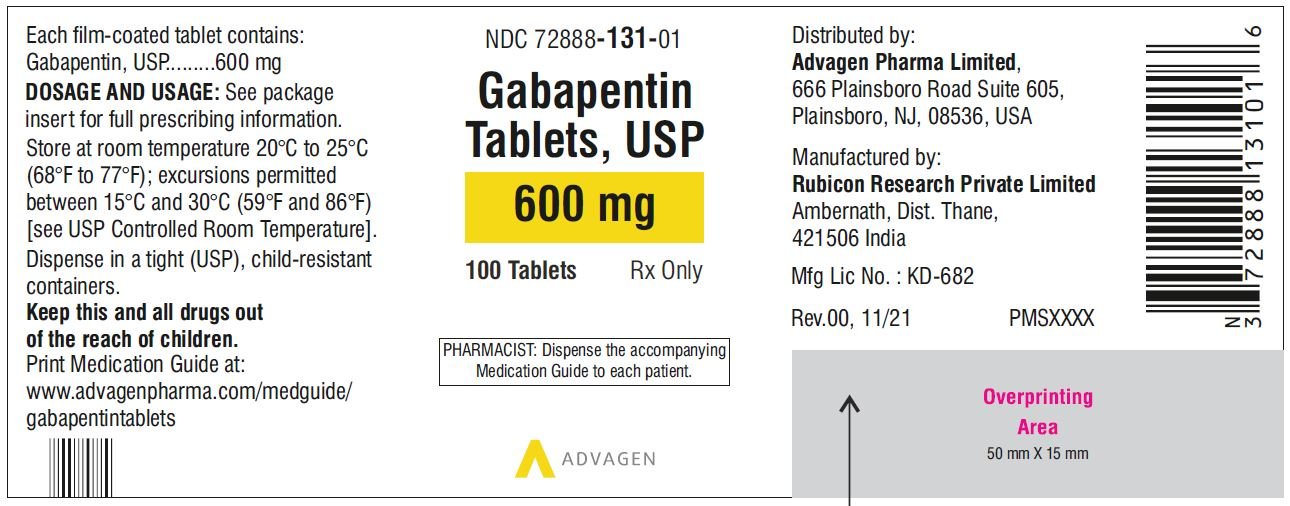
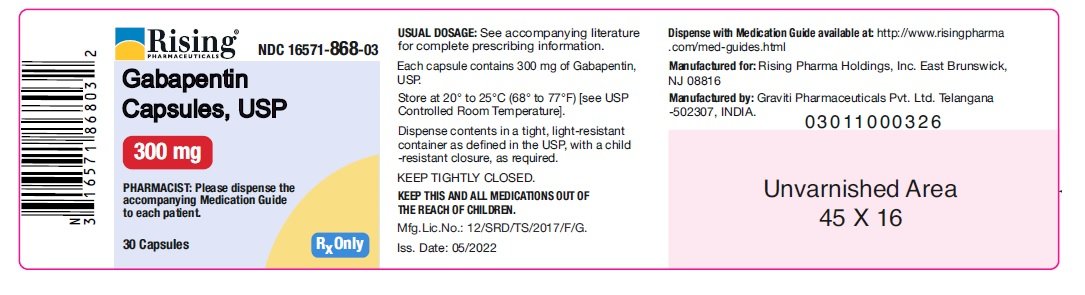
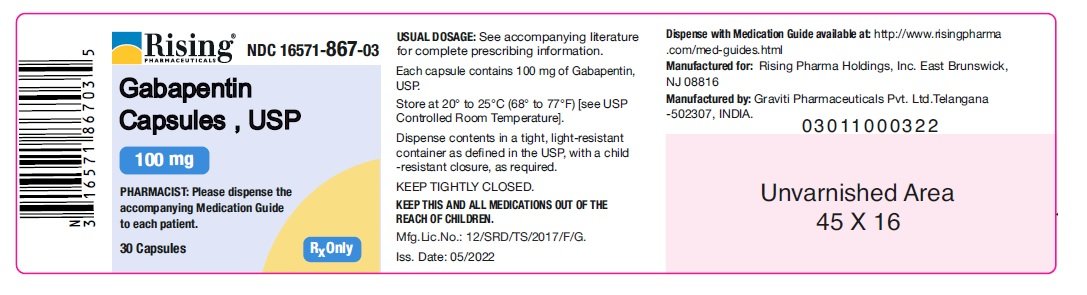
Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. After oral administration, gabapentin enacarbil undergoes extensive first-pass hydrolysis by non-specific carboxylesterases primarily in enterocytes and to a lesser extent in the liver, to form gabapentin, carbon dioxide, acetaldehyde, and isobutyric acid. The unique absorption of Horizant® (gabapentin enacarbil) enables extended and dose-proportional exposure. See Important Safety Information. HORIZANT (gabapentin enacarbil) is a prodrug of gabapentin, an antiepileptic drug (AED). AEDs increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. In nonclinical studies in rat and rabbits, administration of 251 gabapentin enacarbil was developmentally toxic when administered to pregnant animals at doses 252 and gabapentin exposures greater than those used clinically. HORIZANT should be used during 253 pregnancy only if the potential benefit justifies the potential risk to the fetus. HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. 261 2 times the gabapentin exposure associated with the maximum recommended human dose 262 (MRHD) of 1,200 mg/day gabapentin enacarbil on an area under the curve (AUC) basis. 263 When pregnant rabbits were administered gabapentin enacarbil (oral doses of 200, 500, 264 or 2,500 mg/kg/day) throughout the period of organogenesis, embryo-fetal Horizant® (gabapentin enacarbil) offers once daily dosing for RLS and single step titration for PHN. Learn how to dose HORIZANT®. See Important Safety Information. There are savings programs with Horizant® (gabapentin enacarbil) for every patient, regardless of insurance coverage. See Important Safety Information. Active ingredients: gabapentin enacarbil Inactive ingredients: Both the 300 mg and 600 mg tablets contain colloidal silicon dioxide, dibasic calcium phosphate dihydrate, glyceryl behenate, magnesium stearate, sodium lauryl sulfate, and talc. This Medication Guide has been approved by the U.S. Food and Drug Administration. Manufactured for: Download helpful Horizant® (gabapentin enacarbil) resources for Restless Legs Syndrome (RLS) and Postherpetic Neuralgia (PHN). See Important Safety Information. 12.1 Mechanism of Action - Gabapentin enacarbil is a prodrug of gabapentin and, accordingly, its therapeutic effects in RLS and PHN are attributable to gabapentin. The precise mechanism by which HORIZANT (gabapentin enacarbil) Extended-Release Tablets for oral use Initial U.S. Approval: 2011 DESCRIPTION HORIZANT (gabapentin enacarbil) is a prodrug of gabapentin. Gabapentin enacarbil is described as (1- { [ ( { (1RS)-1- [ (2-Methylpropanoyl)oxy]ethoxy}carbonyl)amino]methyl} cyclohexyl) acetic acid. It has a molecular formula of C16H27NO6 and a molecular weight of 329.39. Gabapentin HORIZANT (gabapentin enacarbil) is a prodrug of gabapentin, an antiepileptic drug (AED). AEDs increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Suicidal behavior and ideation have also been reported in patients after discontinuation of gabapentin [see Warnings and Precautions ( 5.5)] . Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in Patients taking gabapentin should not drive until they have gained sufficient experience to assess whether gabapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Gabarone package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Usual Dosing (Adults) INDICATIONS AND USAGE HORIZANT is indicated for: -treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. -management of postherpetic neuralgia (PHN) in adults. DOSAGE AND ADMINISTRATION Instruct patients to swallow tablets whole and not to cut, crush, or chew tablets. Take with food. RLS: 600 mg once daily taken at about 5 PM. -A dose of 1,200 mg Important Safety Information for HORIZANT ® (gabapentin enacarbil) Extended-Release Tablets INDICATIONS: HORIZANT ® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. HORIZANT Gabapentin (Neurontin®, Gralise®) is FDA-approved for use in adults with postherpetic neuralgia (PHN). Neurontin® is also approved as adjunctive therapy for managing partial seizures with or without secondary generalization in patients with epilepsy.1-4 Gabapentin enacarbil (Horizant®) is
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |