Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
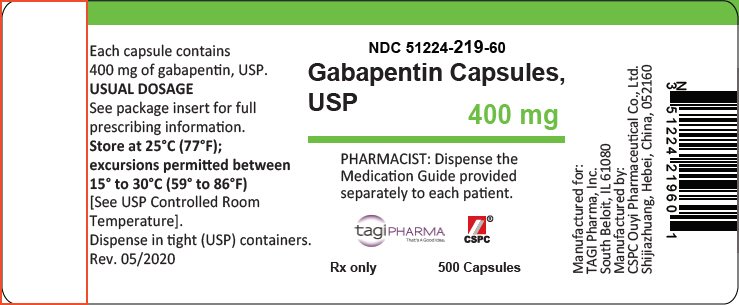 |  |
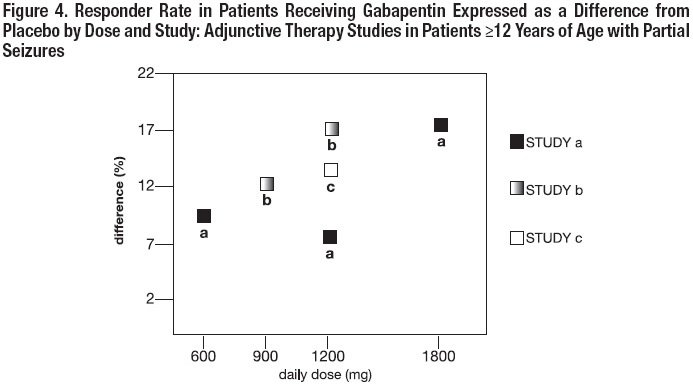
FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) Gabapentin is approved by the United States Food and Drug Administration (U.S. FDA) to treat postherpetic neuralgia and epilepsy with partial-onset seizures. 1 However, much of gabapentin’s use in clinical practice is off label for non–FDA-approved indications. One retrospective evaluation of 105 patient’s reimbursement claims and medical charts found that 95% of patients receive Draft Guidance on Gabapentin November 2023 This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To Analysis of responder rate using combined data from all three studies and all doses (N=162, Neurontin; N=89, placebo) also showed a significant advantage for Neurontin over placebo in reducing the frequency of secondarily generalized tonic-clonic seizures. Gralise (gabapentin) Tablets 300 mg and 600 mg Company: Abbott Products, Inc. Application No.: 022544 Approval Date: 01/28/2011 Persons with disabilities having problems accessing the PDF files below may call (301) 796-3634 for assistance. Approval Letter (s) (PDF) Summary Review (PDF) Officer/Employee List (PDF) Cross Discipline Team Leader During the controlled epilepsy trials in patients older than 12 years of age receiving doses of NEURONTIN up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving NEURONTIN compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Important Limitation: GRALISE is not interchangeable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration (See Warnings and Precautions) Although there have been increasing reports of intentional gabapentin misuse, epidemiological evidence for the phenomenon is limited. The purpose of this study was to determine whether there are pharmacovigilance abuse signals for gabapentin. Using Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba Analysis of responder rate using combined data from all three studies and all doses (N=162, Neurontin®; N=89, placebo) also showed a significant advantage for Neurontin® over placebo in reducing the frequency of secondarily generalized tonic-clonic seizures. When prescribing gabapentin carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., development of tolerance, self-dose escalation, and drug-seeking behavior). During the controlled epilepsy trials in patients older than 12 years of age receiving doses of NEURONTIN up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving NEURONTIN compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for Advise the patient to read the FDA-approved patient labeling (Medication Guide). Administration Information - Inform patients that gabapentin capsules are taken orally with or without DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. 2.1 Dosage for Postherpetic Neuralgia In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In clinical studies, efficacy was Draft Guidance on Gabapentin Enacarbil This draft guidance, once finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Note: If you need help accessing information in different file formats, see . Language Assistance Available: | | | | | | | | | | | | | | |
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |