Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
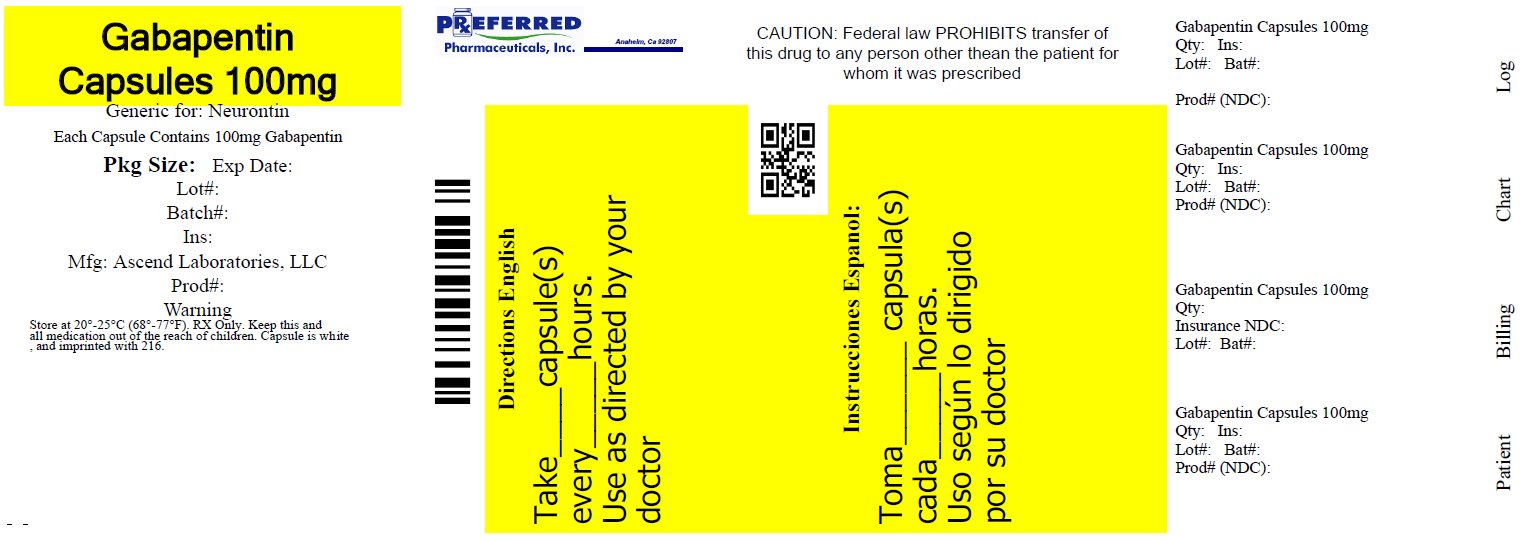
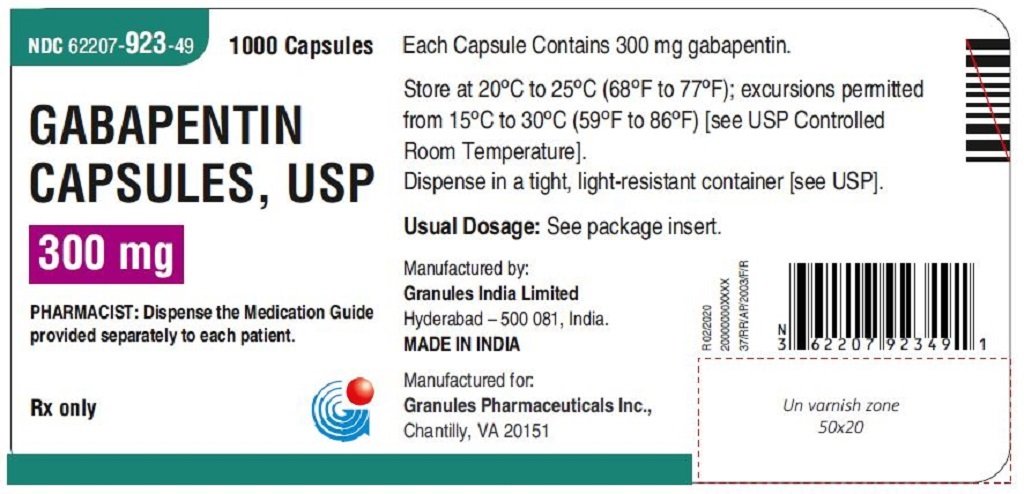
Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Gabapentin is available in both branded and generic forms. Neurontin may be used in combination with other antiepileptic drugs without concern for alteration of the blood concentrations of gabapentin or of other antiepileptic drugs. Important Limitation: GRALISE is not interchangeable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration (See Warnings and Precautions) In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 Gabapentin (brand name Neurontin by Pfizer) is FDA-approved to treat two conditions: an adjunct therapy for partial onset seizures associated with epilepsy and nerve pain after shingles. Other approved uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was first approved in 2004. Due to its calming effects on nerve signaling, it is also used to manage withdrawal symptoms from alcohol and opioids. What Are the Approved Uses of Gabapentin? Approved uses of Gabapentin are the treatment of epilepsy and postherpetic neuralgia, as recognized by the FDA. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Gabapentin is an anticonvulsant (antiseizure) medication approved by the FDA to treat several conditions. Doctors sometimes prescribe gabapentin "off-label" to treat other conditions as well. A 2022 report stated that gabapentin was among the 10 most commonly prescribed medications in the U.S. What is gabapentin and what is it used for? Gabapentin is used to control seizures, to treat nerve NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 Gabapentin is from a group of medicines called anticonvulsants. Different brands of gabapentin are not interchangeable and they are FDA approved for different conditions. Use only the brand and form of gabapentin your doctor has prescribed. Check your medicine each time you get a refill to make sure you receive the correct form. Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was Gabapentin has been approved by the United States (US) Food and Drug Administration (FDA) for postherpetic neuralgia and as adjunctive therapy for focal seizures. 1 However, a recent analysis of US physician office-based prescription practices between 2011 and 2016 found that less than one percent of gabapentin prescriptions are for such indications. 2 In 2020, gabapentin was reported to be There are rare postmarketing reports of individuals experiencing withdrawal symptoms shortly after discontinuing higher than recommended doses of gabapentin used to treat illnesses for which the drug is not approved. There are rare postmarketing reports of individuals experiencing withdrawal symptoms shortly after discontinuing higher than recommended doses of gabapentin used to treat illnesses for which the drug is not approved. Neurontin may be used in combination with other antiepileptic drugs without concern for alteration of the blood concentrations of gabapentin or of other antiepileptic drugs. Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. Overview of FDA-approved uses for pregabalin and gabapentin, highlighting their indications and clinical applications in medical practice. Gabapentin gained FDA approval in 1993 under the brand name Neurontin as an adjunctive therapy in the treatment of partial onset seizures, and subsequently for the treatment of postherpetic neuralgia in adults in 2002. It became available as a generic in 2004. Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |