Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
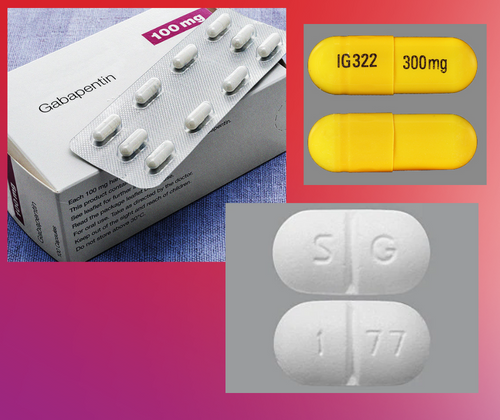
The U.S. Supreme Court on Monday left intact a $142 million jury verdict against Pfizer Inc over the marketing of the epilepsy drug Neurontin. · The purpose of this notice is to alert you to a proposed settlement of a Class Action Lawsuit (the “Lawsuit”) brought by Direct Purchasers of Neurontin against Pfizer Inc. and Warner-Lambert Company LLC (collectively “Pfizer” or “Defendants”). The Lawsuit asserts that Pfizer violated antitrust laws relating to the sale of its prescription drug Neurontin. Neurontin Lawsuits Neurontin (generic name: gabapentin) was FDA-approved in 1993 for use as an anticonvulsant for people suffering from partial seizures associated with epilepsy. Los Angeles, CA: A $325 million preliminary settlement has been reached by Pfizer Inc and Warner-Lambert Co. LLC and plaintiffs who filed a consumer fraud class action lawsuit over the marketing Gabapentin Lawsuit – How to Join The gabapentin class action lawsuit was filed in 2022 against three pharmaceutical companies – Teva, Pfizer, and Greenstone – accusing them of misrepresenting the drug’s risks and overstating its benefits. To join the gabapentin lawsuit, potential class members need to meet the following criteria: Have taken brand name Neurontin or generic gabapentin American pharmaceutical giant Pfizer Inc. and its subsidiary Pharmacia & Upjohn Company Inc. (hereinafter together “Pfizer”) have agreed to pay $2.3 billion, the largest health care fraud settlement in the history of the Department of Justice, to resolve criminal and civil liability arising from the illegal promotion of certain pharmaceutical products. If you purchased one or more eligible HexClad cookware products between February 1, 2022 and March 31, 2024, you may be eligible for a cash payment from a $2.5 million class action settlement Pfizer Inc has agreed to pay $325 million to resolve claims it defrauded insurers and other healthcare benefit providers by marketing Neurontin for unapproved uses, its second settlement over the Redirecting to Pfizer, the world's largest drug maker, pleaded guilty on 13 May to numerous civil and criminal charges for illegally promoting the off-label use of gabapentin (Neurontin). It has agreed to pay a $240m (£136m; €200m) criminal fine and $152m to state and federal healthcare programmes. The fine is the second largest given in the industry. Meanwhile, off-label sales of gabapentin continue to In recent years, the manufacturers of Gabapentin have faced legal challenges as a result of these issues. One notable lawsuit emerged when the manufacturers of Neurontin reached a $325 million class action lawsuit settlement over allegations of fraudulent marketing practices. Consumers are filing lawsuits against prescription drug and medical device companies after facing serious injuries. Find out if you qualify for a lawsuit. If you have suffered an injury after taking Neurontin, you may need to file a Neurontin lawsuit to get compensation. LegalMatch lawyers can help today. We would like to show you a description here but the site won’t allow us. Discover the latest on Gabapentin lawsuits, including settlements, payouts, and claims related to nerve damage, seizures, and off-label uses, with expert guidance on filing a case and seeking compensation for victims of this anticonvulsant medication. An $85 million class action has been reached with Sun Pharmaceutical Industries and Taro Pharmaceuticals. The class action lawsuit claims that certain generic drug manufacturers broke antitrust laws, leading to artificially higher prices for common generic drugs such as amphetamine, amoxicillin, gabapentin, baclofen, and others. A $190 million settlement has been reached in New York in a consumer fraud class action lawsuit pending against Pfizer which alleges the pharma giant engaged in tactics to delay market entry of generic versions of its epilepsy drug Neurontin. The lawsuit was filed by purchasers of Neurontin in 2002, claiming Pfizer And it's the second settlement of Neurontin-related claims in less than 6 weeks. On April 21, Pfizer said it would pay $190 million to settle a federal antitrust lawsuit claiming that the company What’s Next? Eligible FuboTV users are encouraged to act quickly to submit their claims before the September 12, 2025, deadline. The settlement website provides detailed instructions and resources for filing claims. Those who wish to opt out of the settlement or object to its terms must do so by August 28, 2025. Explore the potential for seeking compensation in gabapentin-related memory loss cases, including legal grounds, evidence, and filing deadlines.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |