Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
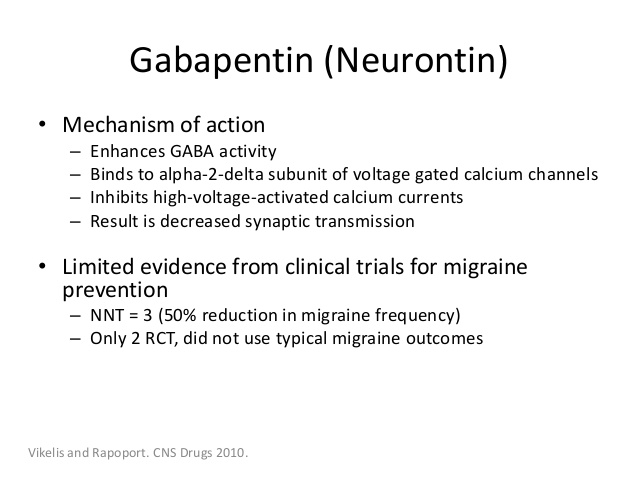
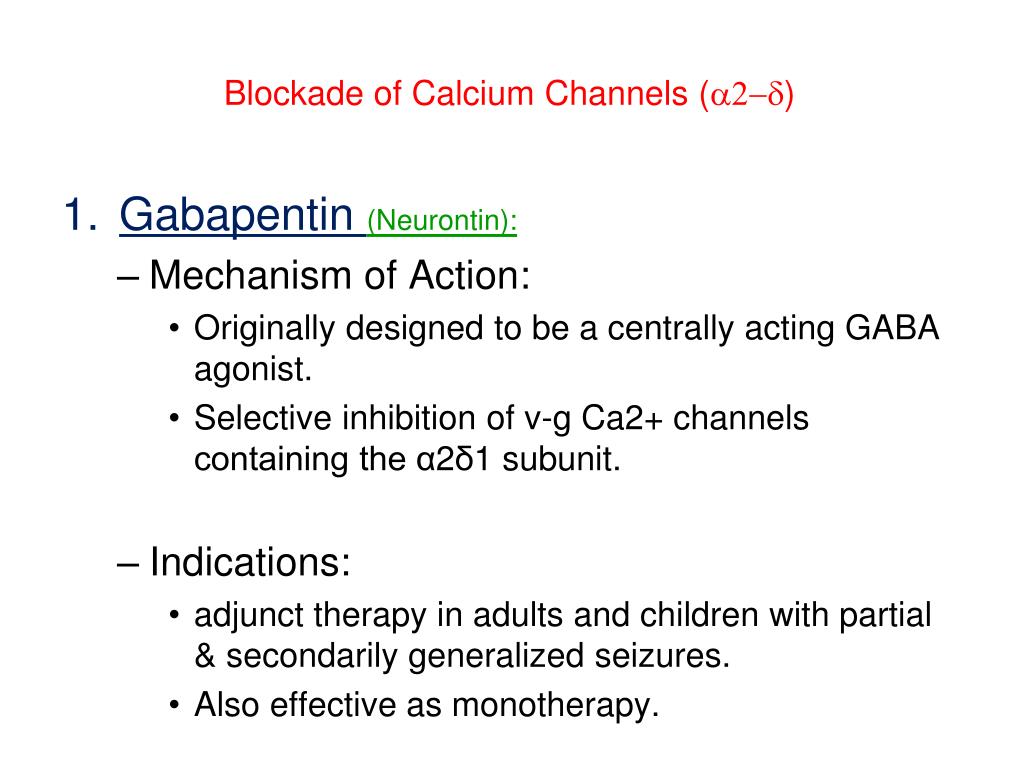
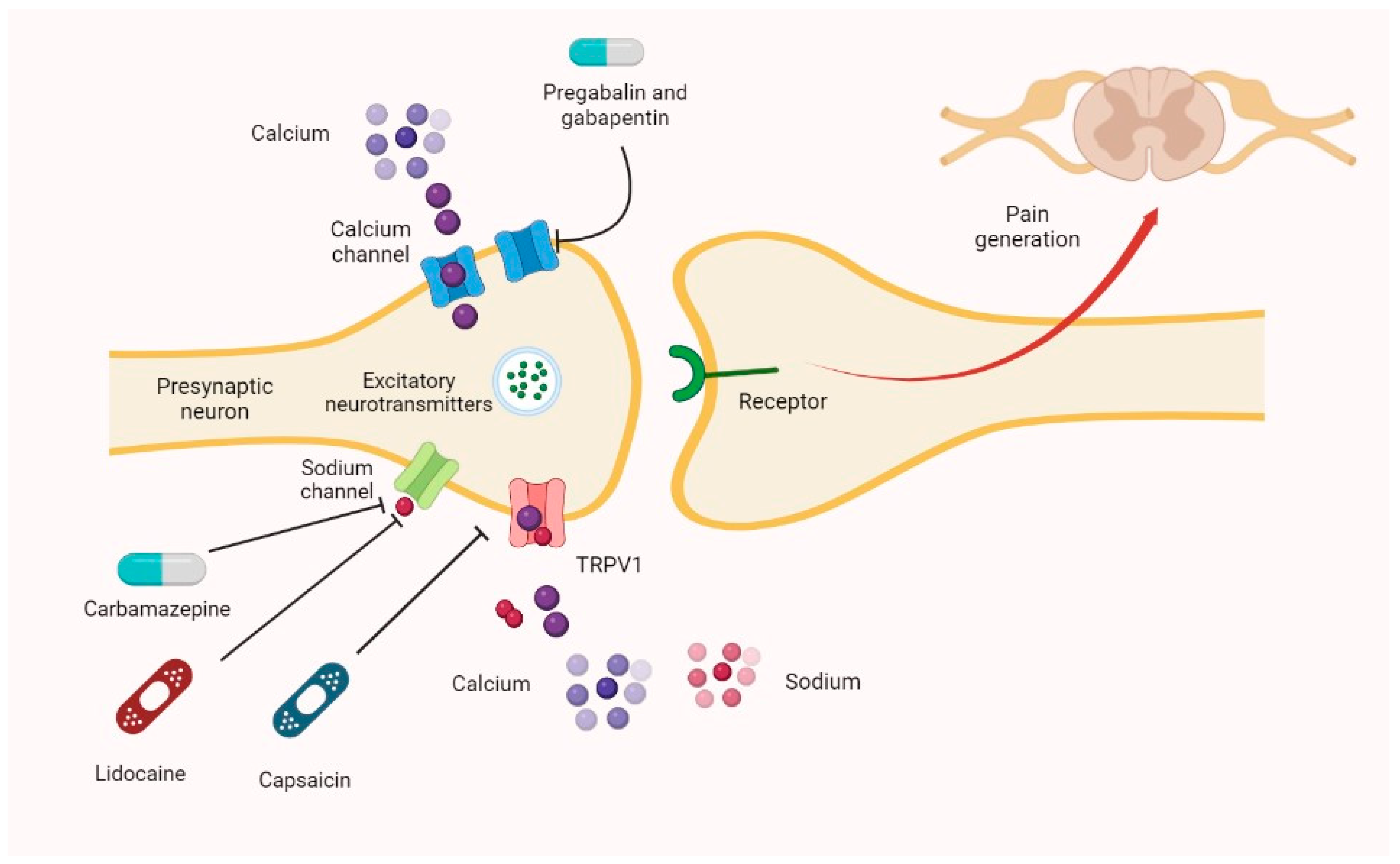
Figure 1. Mechanisms of Gabapentin Antalgic Action: GABA Synthesis and Glutamatergic Inhibition (A) The pathways leading to GABA synthesis and degradation. (B) The analgesic effect of gabapentin depends on the inhibition of excitatory glutamatergic neurons, occurring through mechanisms that do not involve GABA receptors. Gabapentin prevents pain responses in several animal models of hyperalgesia and prevents neuronal death in vitro and in vivo with models of the neurodegenerative disease amyotrophic lateral sclerosis (ALS). Gabapentin is also active in models that detect anxiolytic activity. Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific bindin Here we review the current understanding of the pathophysiological role of the α 2 δ ‐1 subunit, the mechanisms of analgesic action of gabapentinoid drugs and implications for efficacy in the clinic. Despite widespread use, the number needed to treat for gabapentin and pregabalin averages from 3 to 8 across neuropathies. Gabapentin [1- (aminomethyl)cyclohexane acetic acid] is␣a␣novel anti-epileptic agent, originally developed as a gamma-aminobutyric acid (GABA)-mimetic compound to treat spasticity, and has been shown to have potent anticonvulsive effects [1, 2]. Initially approved only for use in partial seizures, it soon showed promise in the treatment of chronic pain syndromes, especially neuropathic General Description Gabapentin is an analgesic medication commonly used for neuropathic pain. It exerts its effects through various mechanisms of action, including the inhibition of voltage-gated calcium channels (VGCCs) and disruption of α2δ-1-NMDAR complexes. By reducing the release of excitatory neurotransmitters and blocking synaptogenesis, gabapentin helps alleviate neuropathic pain. It Introduction Gabapentin is an antiepileptic drug and one of the most widely prescribed medications for neuropathic pain, postherpetic neuralgia, and partial seizures. Originally developed as a GABA analog, it surprisingly does not act directly on GABA receptors. Instead, it binds to voltage-gated calcium channels, altering neurotransmitter release. It is also used off-label for conditions like Here we review the current understanding of the pathophysiological role of the α 2 δ ‐1 subunit, the mechanisms of analgesic action of gabapentinoid drugs and implications for efficacy in the clinic. Despite widespread use, the number needed to treat for gabapentin and pregabalin averages from 3 to 8 across neuropathies. Introduction The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain. 1 Pregabalin is also approved for generalised anxiety disorders in the United Kingdom. The mechanisms of action are still unclear despite their widespread use. Mechanism of Action Although the exact mechanism of action with the GABA receptors is unknown, researchers know that gabapentin freely passes the blood-brain barrier and acts on neurotransmitters. Gabapentin has a cyclohexyl group to the structure of the neurotransmitter GABA as a chemical structure. Although it has a structure similar to GABA, it does not bind to GABA receptors or influence Medline and EMBASE database searches were conducted to identify studies relating to mechanisms of action and effects in experimental animal models of inflammatory and postoperative pain and human models of experimental pain. The effects of gabapentinoids may be attributed to depression of dorsal horn sensitivity through a multitude of mechanisms. Introduction Gabapentin is an antiepileptic drug and one of the most widely prescribed medications for neuropathic pain, postherpetic neuralgia, and partial seizures. Originally developed as a GABA analog, it surprisingly does not act directly on GABA receptors. Instead, it binds to voltage-gated calcium channels, altering neurotransmitter release. It is also used off-label for conditions like Gabapentin has been clearly demonstrated to be effective for the treatment of neuropathic pain in diabetic neuropathy and postherpetic neuralgia. This evidence, combined with its favourable side-effect profile in various patient groups (including the elderly) and lack of drug interactions, makes it an attractive agent. Abstract Gabapentin is a drug that has been widely used in the treatment of chronic pain states. Despite its widespread usage, it is only recently that light has been shed on the mechanism of action of this agent. In the current review, the authors document the pharmacological, biochemical and molecular information that has led to the identification of the α2δ1 auxilliary subunit of voltage Alternatively, gabapentin may reduce α2δ-1 interaction with thrombospondin, an astrocyte-secreted protein, and inhibit new synapse formation (but not already formed synapses) (Eroglu et al., 2009). Nevertheless, this action cannot fully account for the relatively rapid onset of gabapentinoid effects on pain hypersensitivity. The present review discusses the effectiveness of gabapentin in different types of neuropathic pain in preclinical as well in clinical settings and also discusses the possible mechanism of action at different levels including at dorsal root ganglion (DRG) and dorsal horn neurons along with at supra-spinal centres. Gabapentinoids, including gabapentin and pregabalin, are extensively used for treatment of neuropathic pain, restless legs syndrome, and focal seizures. Their efficacy in these disorders is primarily attributed to their effects in inhibiting the functions of the α2δ subunit of presynaptic VGCCs, thereby reducing neurotransmitter release. The gabapentinoids, pregabalin and gabapentin, have been the cornerstone of pharmacological management of neuropathic pain. 1 Despite the widespread use in neuropathic pain, the precise mechanism of action is uncertain. The effect of gabapentinoids in pain are assumed to be because of direct inhibition of voltage gated Ca 2+ channels by binding to its α2δ-1 subunit resulting in reduction of The gabapentinoids, pregabalin and gabapentin, have been the cornerstone of pharmacological management of neuropathic pain.1 Despite the widespread use in neuropathic pain, the precise mechanism of action is uncertain. The effect of gaba-pentinoids in pain are assumed to be because of direct inhibi-tion of voltage gated Ca2þ channels by binding to its a2d-1 subunit resulting in reduction of Gabapentin has become popular as a first-line treatment for neuropathic pain because of its efficacy as an antineuropathic agent and relatively benign side-effect profile. However, its mechanism of action is far from clear. This review discusses the available evidence for the postulated mechanisms of action of gabapentin.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |