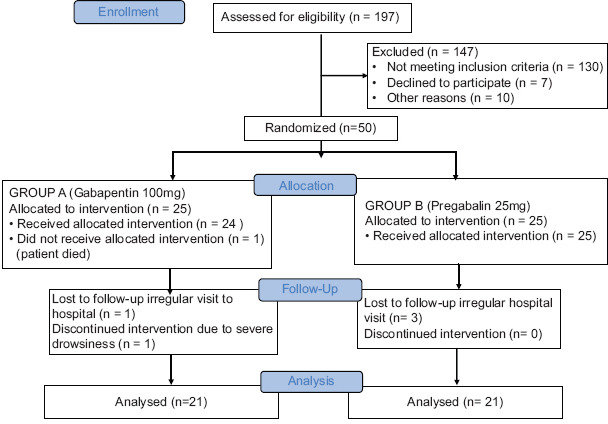
Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
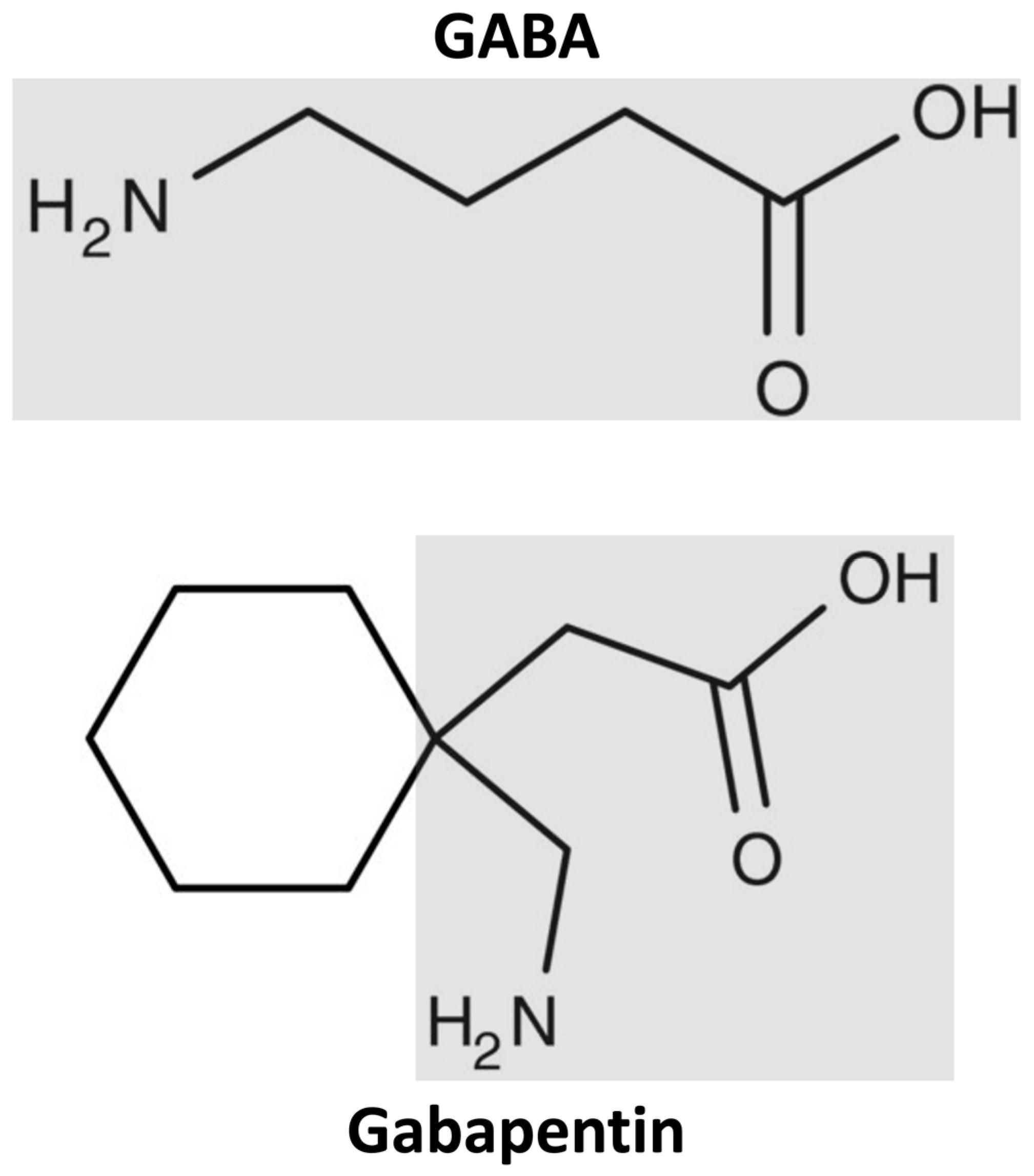
Gabapentin has a low oral bioavailability that is dose-dependent and a relatively short half-life. Together this results in a need for frequent daily dosing (generally three times daily). Gabapentin is predominately renally eliminated as unchanged drug, and therefore renal dosage adjustments are necessary in patients with renal insufficiency. Unlike other drugs, gabapentin is not metabolized in the liver and is solely excreted by the kidneys. Therefore, it is crucial to adjust the dosage in patients with renal insufficiency to avoid severe adverse effects. Renal dose adjustments for gabapentin and pregabalin are ubiquitously evident in the medical literature. All manufacturers for these branded and generic dosage forms list dosing recommendations relative to creatinine clearance (CrCl) for both medications (Table 1). 1,2 However, the basis of these recommendations has not been well articulated. Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. As a result, metabolism-related factors do not necessitate dosage alterations of gabapentin and concomitant AEDs after prolonged therapy. The drug is excreted unchanged in urine; plasma clearance is linearly related to creatinine clearance; and dosage is readily adjusted based on renal function. Abstract Background: Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. Hi, Gabapentin is exclusively excreted by the Kidneys and undergoes no appreciable metabolism by the Liver. As to whether it is toxic to your Kidneys is probably a question that you should be asking your prescribing doctor. Certainly, fluid retention is listed as a possible side effect and it is also known that Gabapentin toxicity is an issue for people with Kidney disorders. Good luck. Accumulation can cause renal failure resulting in adverse effects. Formulations Gabapentin is available in two extended-release formulations in addition to the immediate release: a gastric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neuralgia. Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. Gabapentin is a unique anticonvulsant that is used as adjunctive therapy in management of epilepsy and for neuropathic pain syndromes. Therapy with gabapentin is not associated with serum aminotransferase elevations, but several cases of clinically apparent liver injury from gabapentin have been reported. Gabapentin is almost exclusively cleared by the kidney and thus presents challenges in patients with kidney failure. Gabapentin is known to be effectively cleared by hemodialysis, but the efficiency of clearance by peritoneal dialysis (PD) has not been previously described. We report a case of gabapentin toxicity in a patient on long-term PD who was treated with continuous automated cycling PD There was no evidence of gabapentin metabolism even in subjects with severe renal impairment. In summary, impaired renal function results in higher plasma gabapentin concentrations, longer elimination half-lives, and reduced CL/F and CLR values. Abstract Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady state, it has a half-life of 6-8 h, and is eliminated unchanged by renal route with a Gabapentin enacarbil is converted to gabapentin during absorption, before reaching the systemic circulation. 16 Gabapentin produced by hydrolysis of gabapentin enacarbil is also eliminated by the renal clearance pathway, without further metabolism.16, 17 In patients with normal CrCL (≥60 mL/min), CL R after oral administration of gabapentin The data suggest that dosage adjustment for gabapentin enacarbil is necessary in patients with impaired renal function. Gabapentin enacarbil, 600 mg, seemed to be well tolerated in this small selected population. Pharmacodynamics Mechanisms of action Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated Renal impairment: For patients with renal impairment, gabapentin, which is primarily excreted really and lacks active metabolites, requires dosage adjustment. Therefore, it is crucial to modify both the dose amount and frequency accordingly. Gabapentin doesn’t hurt the liver or kidneys in most cases. However, taking a safe gabapentin dose is important to prevent potential side effects. Gabapentin's elimination involves renal clearance, with minimal metabolism by the liver. Its half-life is typically 5-7 hours, but can vary based on renal function. Clinical factors like dosage, individual metabolism, and renal impairment can influence its clearance. Analytical techniques, such as blood tests, are used to monitor drug levels and assess metabolism.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |