Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
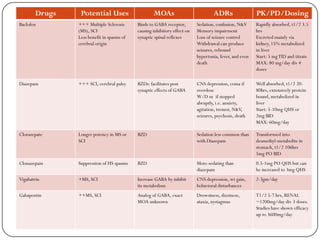 | 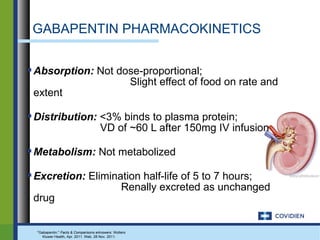 |
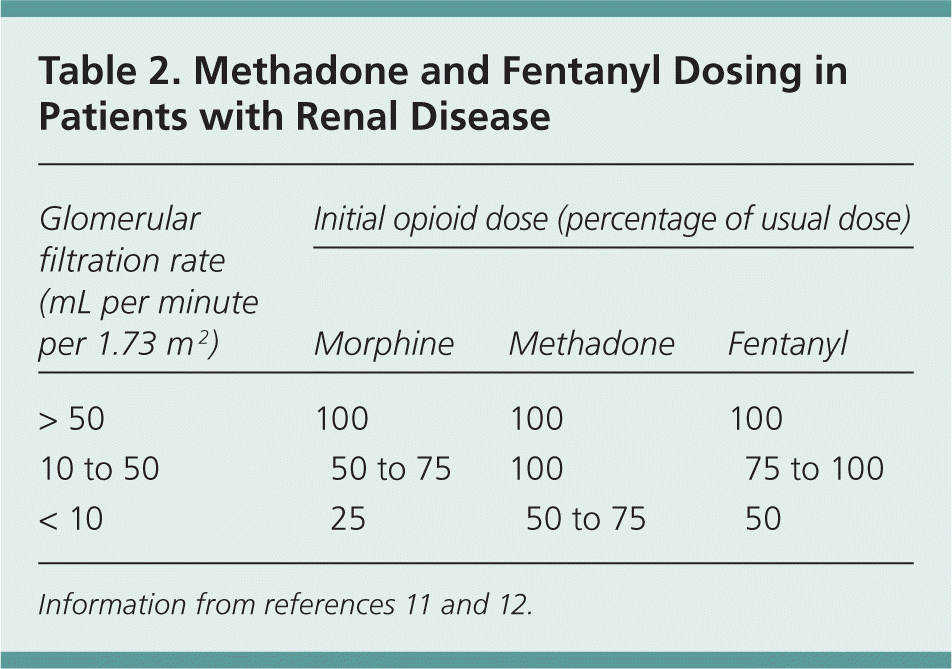
Gabapentin's elimination involves renal clearance, with minimal metabolism by the liver. Its half-life is typically 5-7 hours, but can vary based on renal function. Clinical factors like dosage, individual metabolism, and renal impairment can influence its clearance. Analytical techniques, such as blood tests, are used to monitor drug levels and assess metabolism. Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st Pharmacokinetic properties are important to consider in evaluating the usefulness of new antiepileptic drugs (AEDs). Gabapentin is a new, water-soluble, antiepileptic agent with properties of an amino acid. This drug is rapidly absorbed and exhibits dose-dependent bioavailability as a result of a sa Gabapentin is a unique drug that is not metabolized, does not bind to plasma proteins, and is solely eliminated unchanged by renal excretion [4]. Approximately 76%–81% of a single oral dose is eliminated in the urine and 10%–23% in feces [5]. The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic prof Gabapentin (Trade name: Neurontin) is an anticonvulsant. It is commonly also used off-label for anxiety disorders, restless leg syndrome, and in alcohol use disorder. Gapentin is not metabolized by the liver, and its effects on the liver and kidneys are similar to previous studies. In rare cases, gabapentin can cause DRESS (drug reaction with eosinophilia and systemic symptoms). Pharmacokinetics Not appreciably metabolized in humans Eliminated from the systemic circulation by renal excretion Elimination half-life ≅ 5 to 7 hours In elderly patients and those with impaired renal function, plasma clearance is reduced Mean half-life increased from 6.5 h (CrCl >60 mL/min) to 52 h (CrCL <30 mL/min) Gabapentin can be removed from plasma by hemodialysis Bioavailability is How Is Gabapentin Metabolized? Gabapentin is a tablet, capsule, extended-release (long-acting), and oral solution (liquid) to take by mouth. It is a lipid-soluble drug. The liver does not break down Gabapentin into metabolites, unlike most of the other drugs. Structure of GABA: gabapentin and pregabalin. 10 Pharmacokinetics The actions of gabapentinoids are mainly at an intracellular site and require active uptake. They undergo facilitated transport across cell membranes through system l -amino acid transporters (LAT) as both drugs are structurally similar to the amino acid leucine. The effects of chronic gabapentin are blocked by an inhibitor of Gabapentin isn’t known to harm the liver. It’s not metabolized (broken down) by your liver. While there have been some individual reports of liver damage from gabapentin, it’s considered extremely rare. One uncommon risk with gabapentin is a reaction called DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. Gabapentin is a unique anticonvulsant that is used as adjunctive therapy in management of epilepsy and for neuropathic pain syndromes. Therapy with gabapentin is not associated with serum aminotransferase elevations, but several cases of clinically apparent liver injury from gabapentin have been reported. A lack of hepatic metabolism makes gabapentin an attractive option for patients on multiple antiepileptic drugs and patients with impaired hepatic function. Although gabapentin is relatively well tolerated, patients should be counseled on and monitored for CNS-related adverse effects, including somnolence, dizziness, ataxia, and asthenia. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin is not appreciably metabolized. Gabapentin enacarbil, the prodrug of gabapentin, is rapidly and efficiently converted to gabapentin by first-pass hydrolysis following oral administration. Gabapentin | Deranged PhysiologyGabapentin Impact on Other Organs While gabapentin primarily targets the CNS, its effects ripple through other organ systems as well. The kidneys, liver, and gastrointestinal tract all play roles in how gabapentin is metabolized and eliminated from the body. Effects on the Kidneys Gabapentin is primarily excreted unchanged by the kidneys. After oral administration and absorption, gabapentin circulates essentially unbound to serum proteins. In addition, gabapentin does not undergo hepatic metabolism, unlike most other antiepileptic drugs, and is eliminated almost entirely by renal excretion with a clearance that approximates the glomerular filtration rate. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |