Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 | |
 | 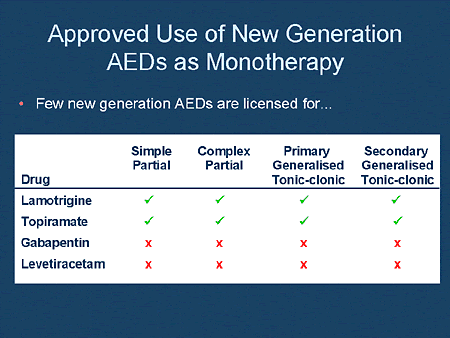 |
The efficacy and safety of gabapentin as monotherapy for treatment of partial onset seizures were evaluated in three large multicenter, double-blind, parallel-group, dose-controlled trials. This is a protocol for a Cochrane Review (Intervention). The objectives are as follows: To assess the effects of gabapentin monotherapy for people with epileptic partial seizures with and without secondary generalisation. Background Epilepsy is one of the most common chronic neurological disorders, affecting more than 50 million people globally. In this review we summarised the evidence from randomised controlled trials of gabapentin used as monotherapy for the treatment of focal epilepsy, both newly diagnosed and drug-resistant, with or without secondary generalisation. Background Monotherapy is the goal for pharmacological treatment of epilepsy. Well-controlled trials have established the efficacy of some of the newer antiepileptic drugs (AEDs) as monotherapy.Objective To review clinical data and expert opinions pertinent to the evaluation of most of The new antiepileptic medications are prescribed for the treatment of patients with seizure disorders since 17 years ago. Gabapentin (GBP) was approved on January 1994 as adjunctive treatment in patients 12 years or older with partial seizures, with Epilepsy is one of the most common chronic neurological disorders, affecting more than 50 million people globally. In this review we summarised the evidence from randomised controlled trials of gabapentin used as monotherapy for the treatment of focal epilepsy, both newly diagnosed and drug-resistant, with or without secondary generalisation. Abstract Background: Gabapentin is widely approved as add-on therapy for epilepsy treatment for partial seizures with and without secondary generalization. To investigate the efficacy of gabapentin administered as monotherapy in patients with newly diagnosed partial epilepsy, a randomized double-blind trial was performed. Objectives To describe the process and results of the updated Swedish practice guidelines for monotherapy in epilepsy. Materials and Methods The Swedish Medical Products Agency led the process tog Learn how first-line antiepileptic drugs, personalized care, and lifestyle changes work together to manage generalized epilepsy effectively. Antiepileptic drug (AED) monotherapy is the preferred initial management approach in epilepsy care, since most patients may be successfully managed with the first or second monotherapy utilized. This article reviews the rationale and evidence Most people with epilepsy are treated with a single antiepileptic drug (monotherapy) and current guidelines from the National Institute for Health and Care Excellence (NICE) in the United Kingdom for adults and children recommend carbamazepine or lamotrigine as first-line treatment for focal onset seizures and sodium valproate for generalised Request PDF | Gabapentin monotherapy for epilepsy: A review | Background: Epilepsy is one of the most common chronic neurological disorders, affecting more than 50 million people globally. In this In patients with partial-onset (focal) seizures, levetiracetam (Keppra), lamotrigine (Lamictal), and carbamazepine (Tegretol) were associated with the longest time to treatment withdrawal (i.e Monotherapy Studies Gabapentin is approved as monotherapy for the treatment of epilepsy in 38 countries. Although gabapentin monotherapy has not been approved in the United States, monotherapy studies provide additional information on gabapentin's efficacy and tolerability. Gabapentin is 1 of many antiseizure medications available for the treatment of epilepsy in adults; however, there are potential risks associated with its use. Therefore, it is important to determine the place of therapy of gabapentin in the treatment of epilepsy. The results showed that gabapentin effectively reduced seizures when used as an additional treatment. Compared to a placebo, gabapentin was almost twice as likely to reduce seizures by 50% or more. The most common side effects associated with gabapentin were ataxia (poor co‐ordination and unsteady gait), dizziness, fatigue and drowsiness. Gabapentin for Seizures: Drugs [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2024 Oct. Table 9, Summary of Recommendations in Included Guidelines. BACKGROUND: Epilepsy is one of the most common chronic neurological disorders, affecting more than 50 million people globally. In this review we summarised the evidence from randomised controlled trials of gabapentin used as monotherapy for the treatment of focal epilepsy, both newly diagnosed and drug-resistant, with or without secondary generalisation. OBJECTIVE: To assess the effects of Abstract Background: Epilepsy is one of the most common chronic neurological disorders, affecting more than 50 million people globally. In this review we summarised the evidence from randomised controlled trials of gabapentin used as monotherapy for the treatment of focal epilepsy, both newly diagnosed and drug-resistant, with or without secondary generalisation. Epilepsy is one of the most common chronic neurological disorders, affecting more than 50 million people globally. In this review we summarised the evidence from randomised controlled trials of gabapentin used as monotherapy for the treatment of focal epilepsy, both newly diagnosed and drug-resistant, with or without secondary generalisation.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 | |
 |  |