Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 | 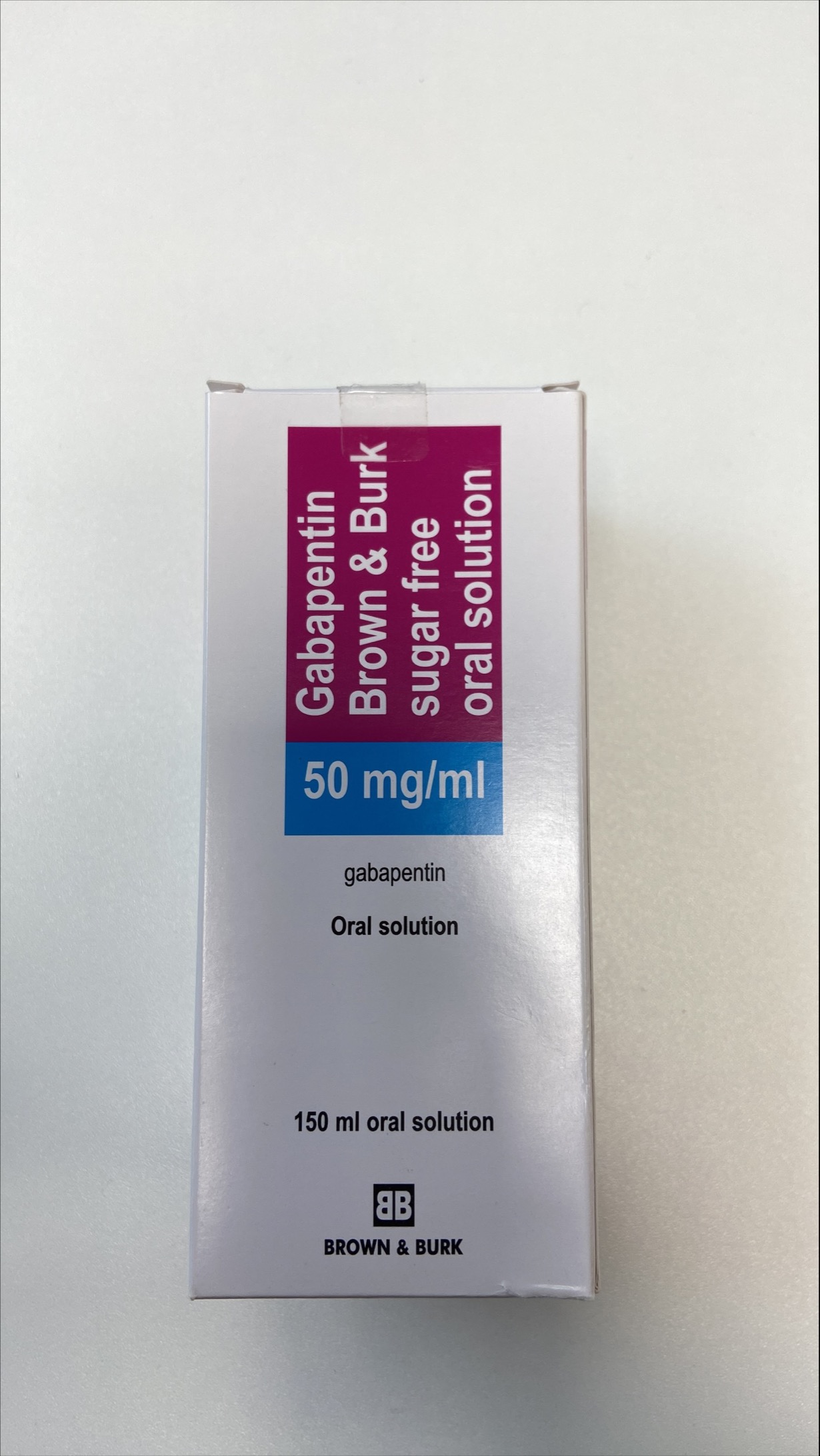 |
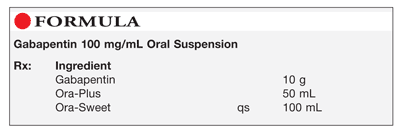
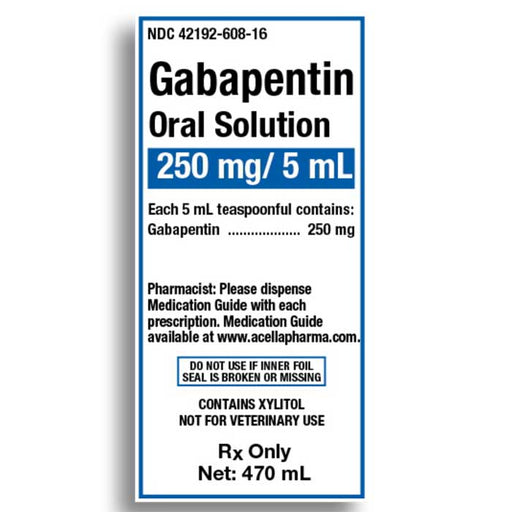
When the decision is made to co-prescribe Gabapentin Oral Solution with another CNS depressant, particularly an opioid, or to prescribe Gabapentin Oral Solution to patients with underlying respiratory impairment, monitor patients for symptoms of respiratory depression and sedation, and consider initiating Gabapentin Oral Solution at a low dose. Gabapentin Oral Solution Stability at Room Temperature Gabapentin oral solution is stable at room temperature for up to 24 hours, according to the American Society of Health-System Pharmacists. After this time frame, it begins to degrade and should be discarded and replaced with a fresh dose. It is important that Gabapentin oral solution not be stored above 25°C (77°F). Liquid Gabapentin Not Gabapentin oral solution is typically available in a liquid form that is designed for oral administration. It contains the active ingredient gabapentin, along with various inactive ingredients to enhance its stability and taste. This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF suspending vehicles As shown in Table 3, the preparation was stable for 91 days at refrigerated and for 56 days at room temperatures. The pH values ranged from pH 5.48 to 5.58. Table 3: Stability of gabapentin 100mg/mL in two oral suspensions in ORA-Plus®/ORA-Sweet® at 4° C and 25° C. Abstract This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF suspending vehicles. Suspensions were packaged in amber plastic bottles and amber plastic syringes at 25°C / 60%RH for up to 90 days. Throughout the study period, the following tests were performed to evaluate DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF suspending vehicles. Suspensions were packaged in amber plastic bottles and amber plastic syringes at 25°C / 60%RH for up to 90 days. Throughout the study period, the following tests were performed to evaluate the Gabapentin Oral Solution can cause serious side effects including: 1. Like other antiepileptic drugs, Gabapentin Oral Solution may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you: Reference: 1. Friciu M, Roullin VG, Leclair G. Stability of gabapentin in extemporaneously compounded oral suspensions. PLoS ONE. 2017; 12(4): 1-11. The Importance of Refrigerating Liquid Gabapentin Understanding Liquid Gabapentin Liquid gabapentin, also known as an oral solution, is prescribed for patients who may have difficulty swallowing tablets or capsules. The liquid formulation of gabapentin is typically mixed with syrup or other liquid substances to make it easier to consume, especially for children or individuals with swallowing This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF suspending vehicles. Suspensions were packaged in amber plastic bottles and clarithromycin [Biaxin]). For more information on storage and stability of reconstituted medications see our chart, Pediatric Oral Antibiotic and Antifungal Suspensions and Liquids (Detail-Documents #231107 [U.S. subscribers] and #231122 [Canadian subscribers]) and our Technician Training Tutorial, Dispensing Pediatric Antibiotic Suspensions. The solution stability of gabapentin in buffered systems was studied in order to facilitate the formulation of a liquid product. The degradation of the drug was followed as a function of pH, buffer concentration, ionic strength, and temperature. Understanding Gabapentin and Its Stability Requirements Gabapentin, a medication primarily used for managing seizures and neuropathic pain, has specific stability requirements that are crucial for ensuring its effectiveness. Improper storage conditions can lead to degradation, impacting its potency and safety. Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin is not available in a liquid dosage form for clinical use. This study was designed to develop two oral gabapentin suspensions and determine their stability under refrigeration or at room temperature. Commercially available gabapentin capsules were used to prepare two suspensions: one in e Gabapentin is available as oral capsules of 100 mg, 300 mg, and 400 mg; tablets of 100 mg, 300 mg, 400 mg, 600 mg, and 800 mg; and an oral solution of 50 mg/mL. However, the need often exists for a higher-concentration oral liquid, and if tablets or capsules are used as the drug source, a suspension will result. This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF suspending vehicles. Suspensions were packaged in amber plastic bottles and When the decision is made to co-prescribe Gabapentin Oral Solution with another CNS depressant, particularly an opioid, or to prescribe Gabapentin Oral Solution to patients with underlying respiratory impairment, monitor patients for symptoms of respiratory depression and sedation, and consider initiating Gabapentin Oral Solution at a low dose.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |