Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
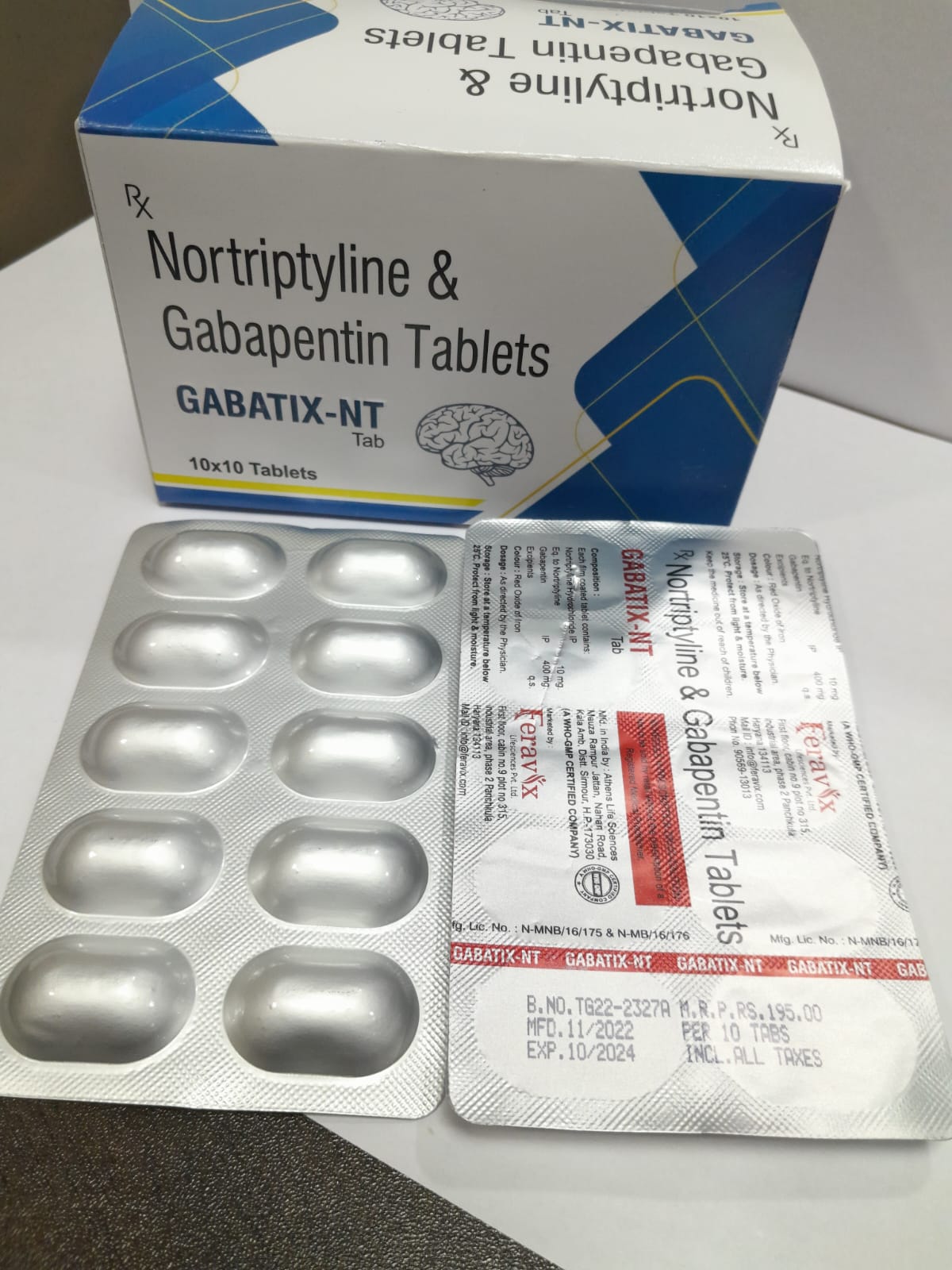
Gabapentin (GBP) is a common name for 1- (aminomethyl)cyclohexaneacetic acid (C 9 H 17 NO 2, CAS Registry No. 60142-96-3), a GABA (γ-aminobutyric acid) derivative (Figure 1) and a popular active pharmaceutical ingredient (API) [1][2]. It has a molecular weight of 171.34 and two pKa values of 3.68 and 10.70 [3][4]. Therefore, at physiological pH, GBP exists in the form of a zwitterion. It was Properties: Crystals from ethanol/ether, mp 162-166° (Satzinger); also reported as mp 165-167° (Schmidt). pKa1 (25°) 3.68; pKa2 10.70. Isoelectric point 7.14. Partition coefficient (octanol/buffer): 0.075 (pH 7.4). Solubility in water at pH 7.4 exceeds 10%. reaction of monomethyl 1, 1-cyclohexanediacetic acid with ethyl chloroformate (dissolved in triethylamine in acetone) and an aqueous solution of sodium azide, the resulting product is refluxed in hydrochloric acid, gabapentin. Showing metabocard for Gabapentin (HMDB0005015) Jump To Section: Identification Taxonomy Ontology Physical properties Spectra Biological properties Concentrations Links References XML enzymes (5) Show 5 proteins According to a classification scheme(2), this estimated Koc value suggests that gabapentin is expected to have high mobility in soil. Experimental pKa values for gabapentin are 3.68 (carboxylic acid) and 10.70 (primary amine)(4), indicating that this compound will primarily exist as a zwitterion in the environment. Gabapentin, USP is a white to off-white powder with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely soluble in water, 0.1 N hydrochloric acid, 0.1 N sodium hydroxide and glacial acetic acid; slightly soluble in methanol, very slightly soluble in ethanol, 2-propanol; insoluble in toluene. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is –1.25. Question: Gabapentin is used to treat some types of seizures, neuropathic pain, and other conditions. It has pKa values of 3.68 and 10.70. Which structures represent the protonation state of gabapentin at pH values of 2, 7, and 12? -NH2. /-NH2 =NH3 NH -NH3 ཁ ལ་ཐག ར ས་པ་ བ ད ས་ ལ་ ་ Gabapentin is a gamma-amino acid that is cyclohexane substituted at position 1 by aminomethyl and carboxymethyl groups. Used for treatment of neuropathic pain and restless legs syndrome. It has a role as an anticonvulsant, a calcium channel blocker, an environmental contaminant and a xenobiotic. It is functionally related to a gamma-aminobutyric acid. Gabapentin is a low molecular weight, polar molecule (log p = −1.1) [24] which exists as a zwitterion at physiological pH [1] and demonstrates two pKa values; 3.68 and 10.70, respectively, for the carboxylic acid and primary amine group (Figure 8) [24]. Gabapentin (GBP) is a common name for 1- (aminomethyl)cyclohexaneacetic acid (C 9 H 17 NO 2, CAS Registry No. 60142-96-3), a GABA (γ-aminobutyric acid) derivative (Figure 1) and a popular active pharmaceutical ingredient (API) [1, 2]. It has a molecular weight of 171.34 and two pKa values of 3.68 and 10.70 [3, 4]. Therefore, at physiological pH, GBP exists in the form of a zwitterion. It was Together, gabapentin generates PKA-dependent pre-synaptic inhibition of GABAergic synaptic transmission, and thereby removes the inhibitory influence on LC neurons only under neuropathic pain states. Veterinary substance properties for Gabapentin, including approvals, environmental fate, eco-toxicity and human health issues Gabapentin (CAS 60142-96-3) information, including chemical properties, structure, melting point, boiling point, density, formula, molecular weight, uses, prices Gabapentin | Deranged PhysiologyGabapentin Chemical Properties Gabapentin (l-[aminomethyl]-cyclohexaneacetic acid) is a new antiepileptic drug related in structure to GABA (Figure). It is fully water soluble, has a molecular weight of 171.34, and a pKa, of 3.68 and a pKa2 of 10.70 at 25°C. Gabapentin freely crosses biologic membranes and can be detected by both gas chromatography and high performance liquid chromatography.1-2 Question: 1. Gabapentin is used to treat some types of seizures, neuropathic pain, and other conditions. It has pKa values of 3.68 and 10.70. Which structures represent the protonation state of gabapentin at pH values of 2, 7, and 12? Gabapentin is a low molecular weight, polar molecule (log p = −1.1) [24] which exists as a zwitterion at physiological pH [1] and demonstrates two pKa values; 3.68 and 10.70, respectively, for the carboxylic acid and primary amine group (Figure 8) [24]. ChemSpider record containing structure, synonyms, properties, vendors and database links for Gabapentin, 60142-96-3, UGJMXCAKCUNAIE-UHFFFAOYSA-N Properties: Crystals from ethanol/ether, mp 162-166° (Satzinger); also reported as mp 165-167° (Schmidt). pKa1 (25°) 3.68; pKa2 10.70. Isoelectric point 7.14. Partition coefficient (octanol/buffer): 0.075 (pH 7.4). Solubility in water at pH 7.4 exceeds 10%. Melting point: mp 162-166° (Satzinger); mp 165-167° (Schmidt) pKa: pKa1 (25°) 3.68; pKa2 10.70 Log P: Partition coefficient (octanol The Gabapentin is small highly polar molecule which can exist as cation, anion and zwitterions due to its acid. pK a of 3.7 and base pKa of 10.7. Initially we tried with ion pairing agent like heptane sulphonic acid and octane sulphonic acid using buffer and organic modifer on C18 column.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |