Gallery
Photos from events, contest for the best costume, videos from master classes.
 | :max_bytes(150000):strip_icc()/soda-recall.jog-289174ba09214d67b5647ddf2bca4529.jpg) |
 |  |
 | |
 |  |
 |  |
 |  |
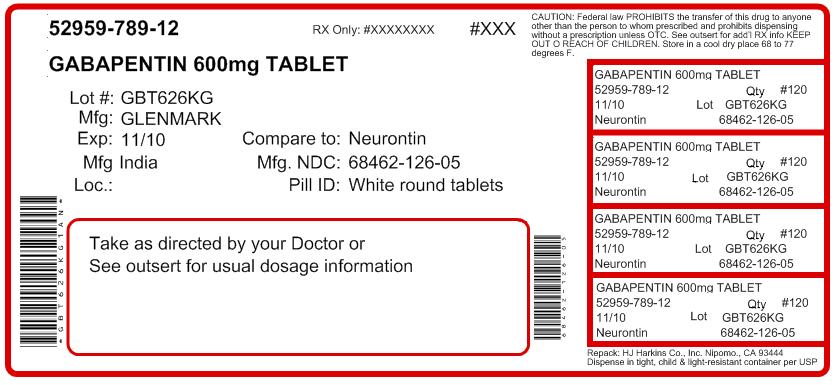
The FDA has reported out that, Sun Pharmaceutical Industries, Inc. initiated a recall for 13,728 bottles of gabapentin, including 12,876 bottles containing 300-milligram dosage and 852 bottles The U.S. Food and Drug Administration (FDA) has reported that Aurobindo Pharma USA is voluntarily recalling Gabapentin 300 mg capsules from lot GESB14011-A in 100-count bottles. The product lot affected was found to include some empty capsules; thus, if taken by a person with seizures, they would Nearly 40 generic medications—most prescription, but some sold over-the-counter at Amazon and Walmart—have been recalled due to manufacturing quality concerns, the FDA said. See the full list GABAPENTIN Recall D-0312-2025 Page Last Updated: April 20, 2025 Home FDA Recalls Class III The Drug Recall Report is for prescription drugs that are have recently been issued recalls by the U.S. Food and Drug Administration (FDA). A US-based subsidiary of Sun Pharma is recalling around 13,700 bottles of Gabapentin capsules, a medication used to treat and prevent seizures in people with epilepsy. FDA Recall Enforcement Reports The most recent Recall Enforcement Report that covers this product was initiated on March 4th, 2025 and classified as a Class III recall due to cross contamination This recall is currently ongoing, and the associated recall number is recall number is D-0312-2025. It pertains to Gabapentin identified by 62756-139. Description: Gabapentin Tablets 600mg, 500-count bottles, Rx Only, Manufactured by: Glenmark Pharmaceuticals Limited, Pithampur, Madhya Pradesh 454775, India, Manufactured for: Glenmark Pharmaceuticals Inc. USA, Mahwah, NJ 07430. The most recent Recall Enforcement Report that covers this product was initiated on July 31st, 2024 and classified as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets This recall is currently terminated, and the associated recall number is recall number is D-0634-2024. It pertains to Gabapentin identified by The latest safety updates on drugs - including duloxetine recall, new warnings for gabapentin-type drugs, tetracycline new warnings, and more. A US-based subsidiary of Sun Pharma is recalling around 13,700 bottles of Gabapentin capsules, a medication used to treat and prevent seizures in people with epilepsy. New Jersey-based Sun Pharmaceutical Industries Inc is recalling the affected lot due to 'Cross Contamination', the USFDA said. The company initiated the Class III recall on March 4, 2025. The US health regulator said Zydus Quantity: 500 or 1,000 capsules NDCs: 62756-139-05, 62756-139-04 Lot numbers: HAD1712B, Exp. date 03/2025; HAD1712C, Exp. date 03/2025 The FDA has classified the recall as a Class III risk, meaning it’s “unlikely to cause health problems but fail to comply with FDA labeling or manufacturing regulations.” For daily wellness updates, subscribe to The Healthy by Reader’s Digest newsletter Glenmark Pharmaceuticals recalls nearly 40 lots of generic medications over manufacturing issues impacting treatments for common health conditions. The recall affects 13 728 bottles—12 876 bottles of 300 mg capsules and 852 bottles of 400 mg capsules—due to concerns of cross contamination during manufacturing. The impacted lots, with expiration dates ranging from March to August 2025, were distributed nationwide across the United States. Sun Pharma’s US subsidiary recalled 13,700 bottles of Gabapentin capsules, a seizure medication, on March 4, 2025, due to cross-contamination concerns. The company classified this as a Class III recall. The Harvard Drug Group, LLC d/b/a Major® Pharmaceuticals and Rugby® Laboratories is initiating a recall of Gabapentin Capsules, USP, 100 mg. This recall has been initiated due to receiving reports of inadequately sealed blister packaging. We have not received any reports of patient harm or adverse events in relation to this issue. Shipping of this product began on December 9, 2024. Multi event Drug Recall Enforcement Report Class III voluntary initiated by SUN PHARMACEUTICAL INDUSTRIES INC, originally initiated on 03-04-2025 for the product Gabapentin Capsules, USP 300 mg, Rx Only, Packaged in a) 500-count bottles, NDC 62756-138-05; b) 1000-count bottles, NDC 62756-138-04, Distributed by: Sun Pharmaceutical Industries, Inc. Cranbury, NJ 08512, Manufactured by: Sun Recall Enforment Report D-0342-2025 Recall Details Multi event Drug Recall Enforcement Report Class II voluntary initiated by Glenmark Pharmaceuticals Inc., USA, originally initiated on 03-13-2025 for the product Diltiazem Hydrochloride Extended-Release Capsules 12HR 120mg, 100-count bottle, Rx, Only. The company initiated the Class III recall on March 4, 2025. The US health regulator said Zydus Pharmaceuticals (USA) Inc is recalling 3,144 bottles of chlorproMAZINE Hydrochloride Tablets, USP 10 mg, a medication used to treat mental health conditions, like schizophrenia and bipolar disorder.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | :max_bytes(150000):strip_icc()/soda-recall.jog-289174ba09214d67b5647ddf2bca4529.jpg) |
 |  |
 | |
 |  |
 |  |
 |  |