Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
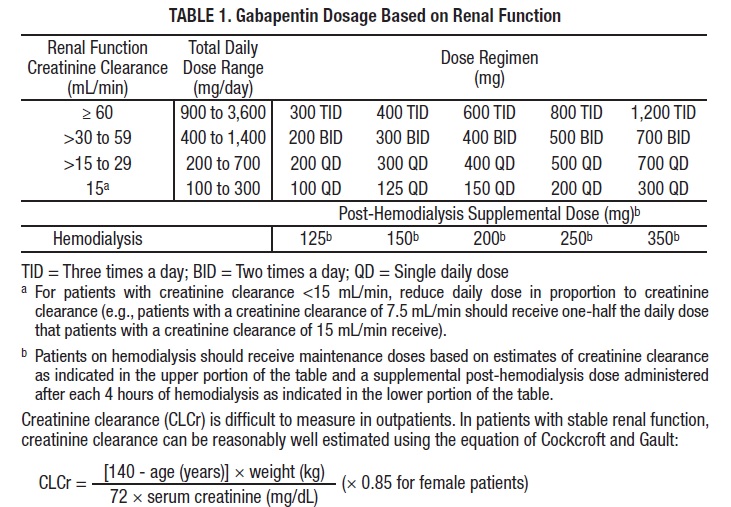
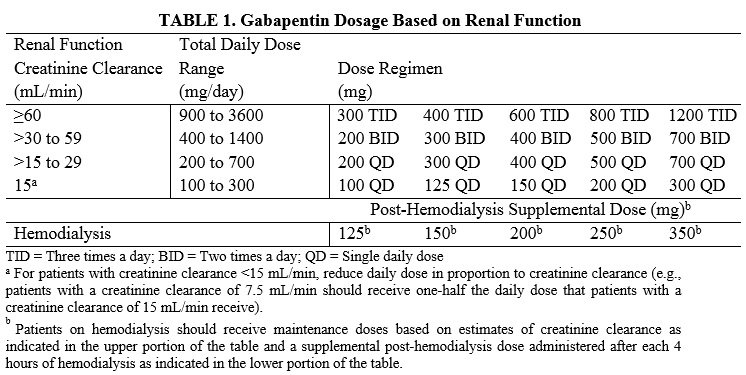
(a) Schedule V consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. This annual publication of the Texas Schedules of Controlled Substances was signed by Jennifer A. Shuford, M.D., M.P.H., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. While gabapentin is not a Federal Schedule V Controlled Substance, these recent actions taken by individual states should alert pharmacists about poten- tial risks related to the drug and high- light the possible need for additional patient counseling in some cases. (5) Pseudoephedrine as an exempt over-the-counter Schedule V controlled substance distributed in the same manner as set forth in Code Section 16-13-29.2; provided, however, that such exemption shall take effect immediately and shall not require rule making by the State Board of Pharmacy; provided, further, that wholesale drug distributors Gabapentin is prescribed frequently for many symptoms and often in an attempt to minimize the use of opioid pain medications. Read more.. Gabapentin is chemically known as 2-[1-(aminomethyl) cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. G.S. 90-93 Page 1 (d) A Schedule V substance may be sold at retail without a prescription only to a person at least 18 years of age. The pharmacist must require every retail purchaser of a Schedule V substance to furnish suitable identification, including proof of age when appropriate, in order to purchase a Schedule V substance. In addition, prescribers need to be aware of two other important changes as follows: R 338.3125 - Gabapentin has been added to the schedule 5 drug list as a controlled substance. As a result of this change, any prescribers prescribing gabapentin must be registered with the Michigan Automated Prescription System (MAPS). Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. Gabapentin – or Neurontin – is a medication commonly used to treat nerve pain and seizures. However, the drug can have potentially harmful effects when combined with other opioids. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Talk to your healthcare provider about the risks of gabapentin before taking it. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. We would like to show you a description here but the site won’t allow us. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. § 54.1-3454. Schedule V. The controlled substances listed in this section are included in Schedule V: 1. Any compound, mixture, or preparation containing limited quantities of any of the following narcotic drugs, which also contains one or more nonnarcotic active medicinal ingredients in sufficient proportion to confer upon the compound, mixture, or preparation, valuable medicinal qualities Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. The schedule depends on the drug’s potential for abuse or dependency. Schedule V drugs have a lower potential for abuse than all other controlled substances. This means gabapentin has a lower risk of abuse compared to Oxycontin (oxycodone), which is a schedule II opioid medication. 50-32-232. Specific dangerous drugs included in Schedule V. Schedule V consists of the drugs and other substances, by whatever official, common, usual, chemical, or brand name designated, listed in this section. (1) Narcotic drugs containing nonnarcotic active medicinal ingredients. Any compound, mixture, or preparation containing any of the following is a narcotic drug, including its salts 2022 Georgia Code Title 16 - Crimes and Offenses Chapter 13 - Controlled Substances Article 2 - Regulation of Controlled Substances Part 1 - Schedules, Offenses, and Penalties § 16-13-29. Schedule v
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |