Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
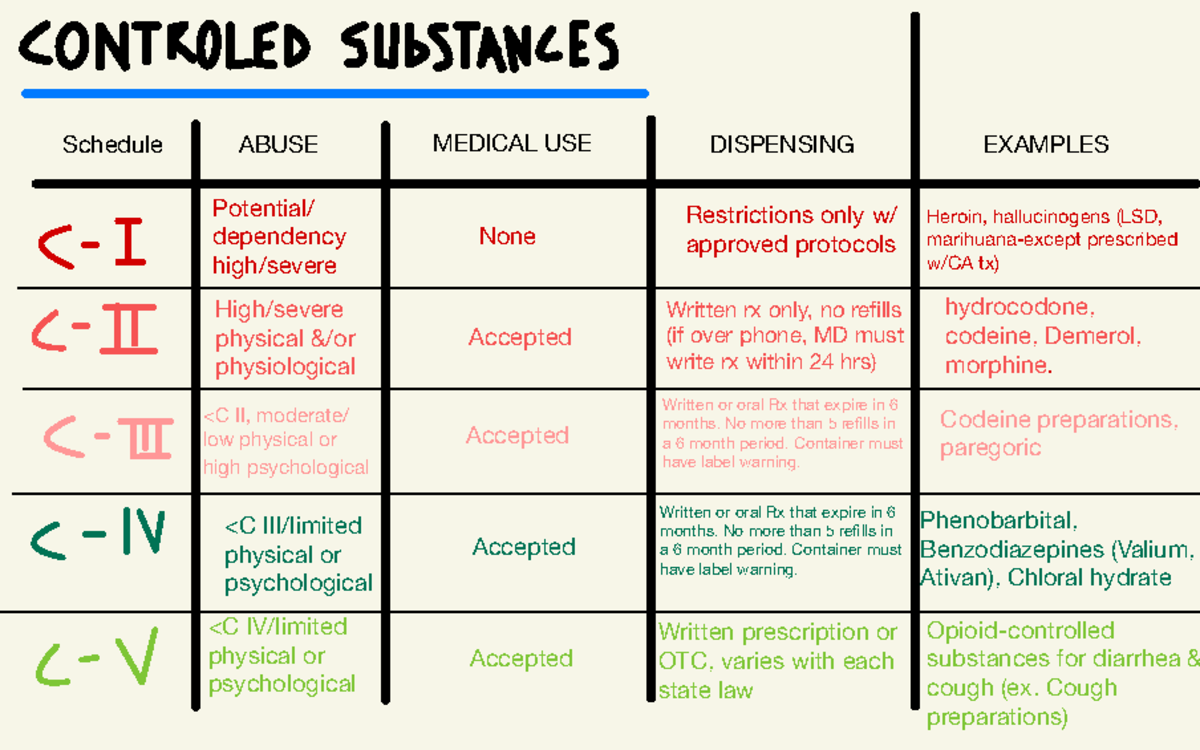
Information on drug and health products authorized by Health Canada. SCHEDULE I (Sections 2, 4 to 7.1, 10, 29, 55 and 60) 1 Opium Poppy (Papaver somniferum), its preparations, derivatives, alkaloids and salts, including: In June 2022, Resolution 514-A-22 (now 13 policy D-120.927) was adopted by the House of Delegates which called upon the AMA to oppose 14 this petition and any other efforts to schedule gabapentin and its salts pending review of the risk 15 and benefits of gabapentin use in the general public and those with substance use disorders. Table of Contents Controlled Drugs and Substances Act 1 - Short Title 2 - Interpretation 4 - PART I - Offences and Punishment 4 - Particular Offences 9 - Report to Parliament 10 - Sentencing 10.1 - PART I.1 - Evidence-based Diversion Measures 10.1 - Principles 10.2 - Warnings and Referrals 10.7 - Exception for Service Providers 11 - PART II Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. SCHEDULE IV (Sections 2, 4 to 7.1, 10, 29, 55 and 60) 1 Barbiturates, their salts and derivatives including (1) All legislative documents related to controlled substances are contained in the Controlled Drugs and Substances Act, the Narcotic Control Regulations, Parts G and J of the Food and Drug Regulations , and the Benzodiazepines and Other Targeted Substances Regulations. 1 NEURONTIN® (Gabapentin Capsules, 100 mg, 300 mg and 400 mg; Gabapentin Tablets, 600 mg and 800 mg), submission control 275525, Product Monograph, BGP Pharma ULC. SCHEDULE IX (Sections 2 and 60) 1. Manual, semi-automatic or fully automatic device that may be used to compact or mould powdered, granular or semi-solid material to produce coherent solid tablets 2. Manual, semi-automatic or fully automatic device that may be used to fill capsules with any powdered, granular, semi-solid or liquid material 2017 Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Importing and exporting 6 (1) Except as authorized under the regulations, no person shall import into Canada or export from Canada a substance included in Schedule I, II, III, IV, V or VI. Marginal note: Possession for the purpose of exporting (2) Except as authorized under the regulations, no person shall possess a substance included in Schedule I, II, III, IV, V or VI for the purpose of Key takeaways: Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule Controlled Drugs and Substances Act (S.C. 1996, c. 19) Full Document: HTML (Accessibility Buttons available) | XML [387 KB] | PDF [706 KB] Briefing Note From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. The status confirmation process includes a scientific review with respect to the heading in the Act for the relevant CDSA Schedule entry. Controlled substances can be used improperly, resulting in harm to public health or safety. The Controlled Drugs and Substances Act (French: Loi réglementant certaines drogues et autres substances) is Canada 's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien 's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled Information on drug and health products authorized by Health Canada.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |