Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
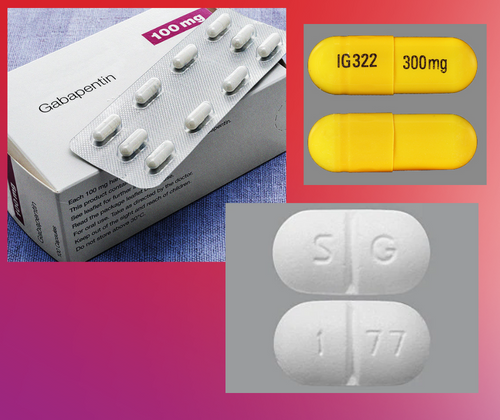
Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Section 23: Written or electronic prescriptions; requirements and restrictions Section 23. (a) A written or electronic prescription for a controlled substance in Schedule II shall become invalid 30 days after the date of issuance. (b) A written or electronic prescription for a controlled substance in schedule II shall not be refilled. Written prescriptions for a controlled substance in Section 2: Establishment of schedules of drugs or other controlled substances Section 2. (a) For the purpose of administration and regulation of the manufacture, distribution, dispensing, and possession of controlled substances by persons authorized under this chapter, the commissioner shall establish by regulations pursuant to the provisions of chapter thirty A five schedules incorporating Classes of controlled substances; establishment of criminal penalties for violations of this chapter Learn how Massachusetts classifies controlled substances, the factors influencing drug schedules, and the legal implications of each classification. Section 31: Classes of controlled substances; establishment of criminal penalties for violations of this chapter Section 31. For the purposes of establishing criminal penalties for violation of a provision of this chapter, there are established the following five classes of controlled substances: CLASS A (a) Unless specifically excepted or unless listed in another schedule, any of the Learn how Massachusetts classifies controlled substances, the factors influencing drug schedules, and the legal implications of each classification. The MA PMP reporting requirements apply to all pharmacies registered with the Massachusetts Board of Pharmacy and to all pharmacies in health facilities registered with the MA DPH that dispense federally controlled substances in Schedules II–V and gabapentin. In addition, the MA PMP reporting requirements apply to any pharmacy located in another state, commonwealth, district, or territory Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Online CME, Massachusetts Medical Society, “Identifying Drug Dependence” (FREE) Utilizing MassPAT Massachusetts law requires that physicians must utilize the prescription-monitoring program prior to prescribing to a patient each time: a narcotic drug in Schedule II or III, and/or a benzodiazepine; OR any controlled substance in Scheduled IV or V which the Department has designated in Section 2: Establishment of schedules of drugs or other controlled substances Section 2. (a) For the purpose of administration and regulation of the manufacture, distribution, dispensing, and possession of controlled substances by persons authorized under this chapter, the commissioner shall establish by regulations pursuant to the provisions of chapter thirty A five schedules incorporating CLASS A (a) Unless specifically excepted or unless listed in another schedule, any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters and ethers, whenever the existence of such isomers, esters, ethers and salts is possible within the specific chemical designation: (1) Acetylmethadol (2 The purpose of this Manual is to provide guidance to registered prescribers and pharmacies1 for prescribing and dispensing controlled substances and medical devices by electronic prescription (ePrescription), as outlined in state law2 and the Department’s ePrescribing regulation.3 Please visit the Department’s website and review the “Massachusetts Request for Temporary Waiver of Daily Data Submission- Gabapentin” waiver form for more information. Reporting Considerations We understand that some prescribers of Gabapentin may not have a personal Drug Enforcement Administration (DEA) number. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). During the period in which you hold only your Massachusetts Controlled Substances Registration, you may conduct approved activities only with Schedule VI controlled substances. You must get both your DEA number and Massachusetts number to conduct approved activities with controlled substances in Schedules II through V. We would like to show you a description here but the site won’t allow us. Who must report? Every pharmacy registered with the Commissioner, that dispenses a controlled substance pursuant to a prescription in Schedules II through V, and Gabapentin (105 CMR 700.012 (7)), and any pharmacy in another state, commonwealth, district or territory that delivers such a controlled substance to a person in Massachusetts. Identification Requirements for CS Prescriptions pharmacy that dispenses federally designated con-trolled substances (CS) and Schedule VI prescription monitoring program (PMP) drugs (eg, gabapentin) is re-quired to check that the photo identification (ID) matches the customer taking possession of the prescription, and that the ID is valid and not out of date.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |