Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 | 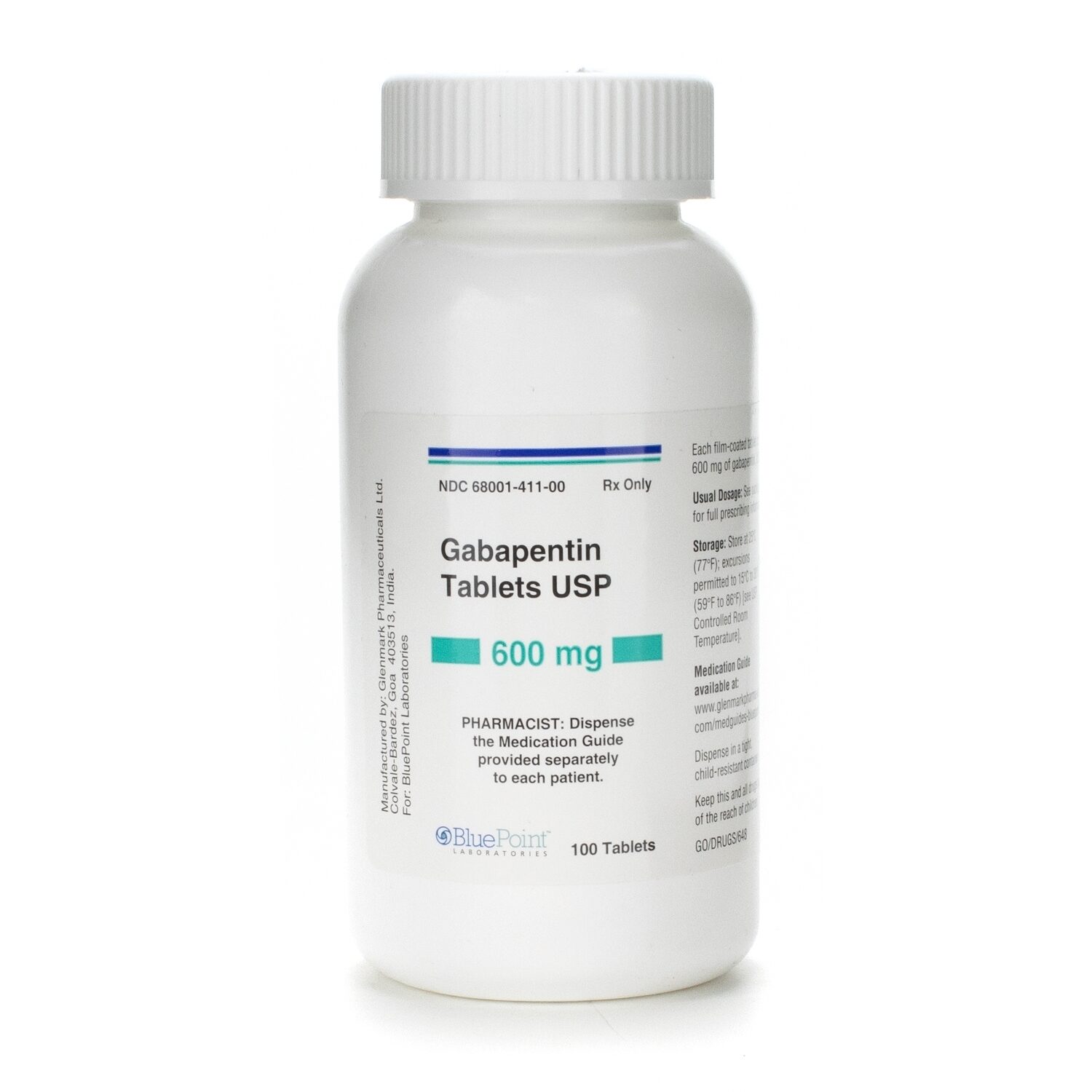 |
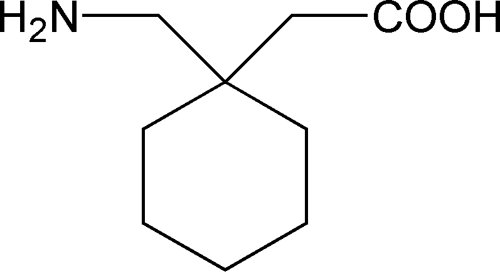
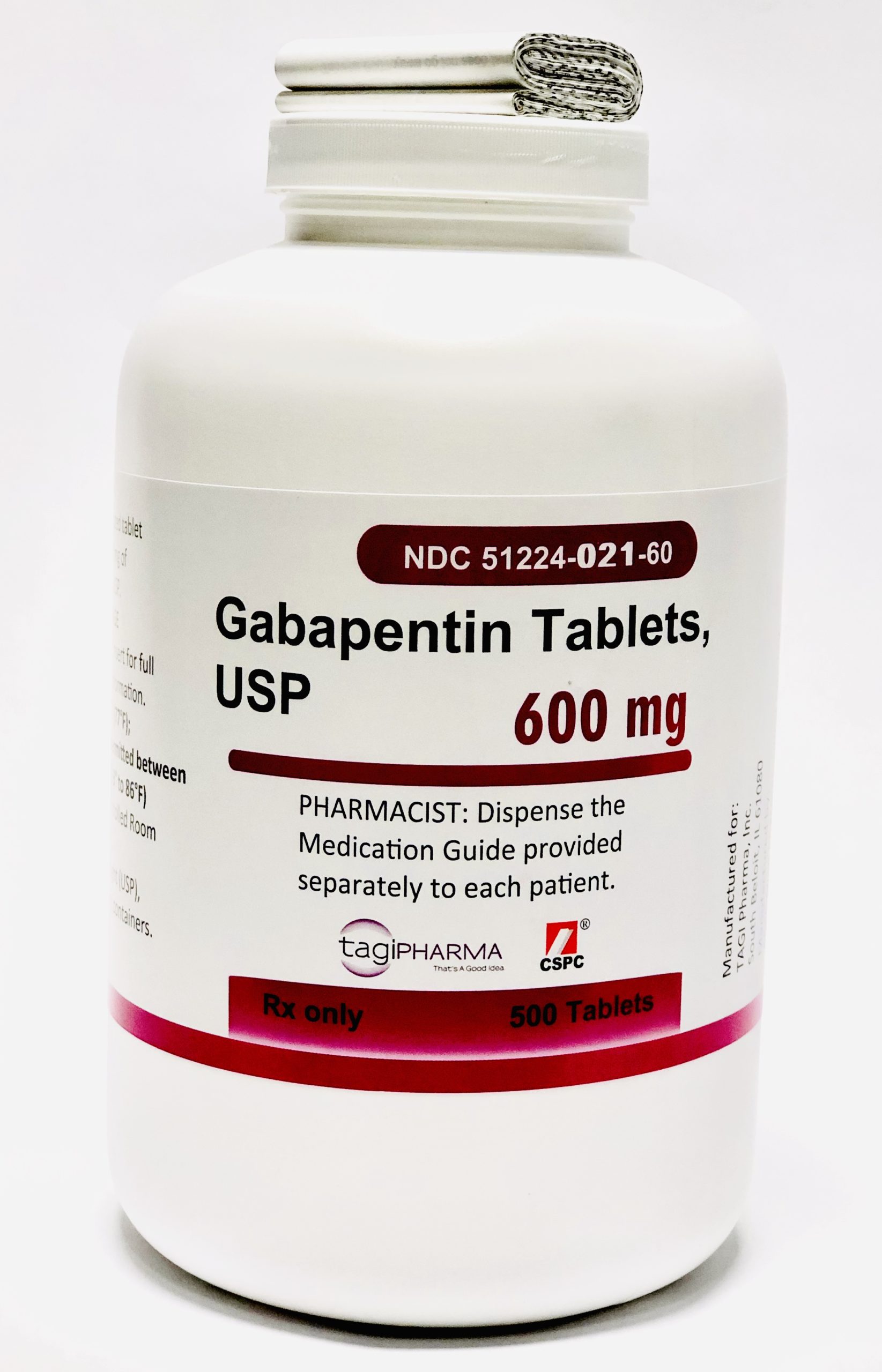
Submission Control No.: 288871 GABAPENTIN (Gabapentin Tablets USP, 600 mg and 800 mg) Page 1 of 35 Our Products Gabapentin Tablets USP 600 mg – 500/Bottle Overview Reference Brand: Neurontin® Therapeutic Class: Antiepileptic Each Gabapentin Tablet, USP contains 600 mg or 800 mg of gabapentin, USP and the following inactive ingredients: corn starch, copovidone, poloxamer 407, magnesium stearate, opadry white, polyethylene glycol and talc. Gabapentin Tablets, USP are supplied as follows: Gabapentin Tablets USP, 600 mg are white to off-white, film-coated, oval shaped, biconvex scored tablets debossed with “G” and “31” on one side Imprint Side 1: G | 31 [Score Line] Imprint Side 2: Score Line Description: White to Off-White, Oval Tablets Strength: 600 mg Available Sizes [NDC & Pack]: 68462-0126-01 100-Count Bottle Our Products Gabapentin Tablets USP 600 mg – 100/Bottle Overview Reference Brand: Neurontin® Therapeutic Class: Antiepileptic Gabapentin tablets, USP 600 mg are available for oral administration as white to off -White, film-coated, oval-shaped tablets, deep scoring on both the sides and debossed with "NT"and “150” on one side supplied in bottles of 100 (NDC – 76267-150-02). Horizant (gabapentin enacarbil) is an extended release tablet used to treat restless legs syndrome and for the pain from having shingles (postherpetic nerve pain). Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older Warnings 100 mg, 300 mg, 400 mg oral capsules 250 mg/5 mL oral solution 100 mg, 300 mg, 400 mg, 450 mg, 600 mg, 750 mg, 800 mg, 900 mg oral tablets How should I store gabapentin? Oral Tablet and Oral Capsule. Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch, copovidone, poloxamer 407, magnesium stearate, polyethylene glycol, talc, hypromellose, titanium dioxide, macrogol, polysorbate 80 and purified water. Gabapentin Tablets USP, 600 mg are white to off-white having mottled spots, oval-shaped, biconvex, film-coated tablets debossed with 'ZE72' with bisect on one side and plain with bisect on other side. Gabapentin film-coated tablet, USP for oral administration contains 100 mg, 300 mg, 400 mg, 600 mg and 800 mg of gabapentin. In addition, each tablet contains the following inactive ingredients: copovidone, hydroxypropyl cellulose, hypromellose, magnesium stearate, polyethylene glycol and titanium dioxide. Gabapentin tablets, USP 600 mg are available as white elliptical film-coated functionally scored tablets debossed with "7" and "5" on one side. Tablets are supplied in bottles of: Bottles of 100 130mm Each film-coated tablet contains 600 mg of gabapentin, USP. USUAL DOSAGE See package insert for full prescribing information. Store at 25°C (77°F); The starting dose is 300 mg three times a day. The recommended maintenance dose of gabapentin tablets is 300 mg to 600 mg three times a day. Dosages up to 2400 mg/day have been well tolerated in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration, and have been well tolerated. Administer gabapentin tablets Gabapentin Tablets, USP 600 mg: White colored film coated, Modified Capsule shaped, biconvex tablets debossed with '1' on the left side of the bisect and '2' on the right side of the bisect on In adults with postherpetic neuralgia, gabapentin tablets may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In clinical studies, efficacy was demonstrated over a range of DESCRIPTION Gabapentin tablets, USP are white colored film coated, modified capsule shaped biconvex tablets containing 600 mg and 800 mg of gabapentin, USP. Each tablet for oral administration contains the following inactive ingredients: Mannitol, Hydroxypropyl Cellulose, Crospovidone, Talc, Magnesium Stearate and Aquarius ® BP18114 Cool Vanilla. Easy-to-read patient leaflet for Gabapentin Tablets 600 mg and 800 mg. Includes indications, proper use, special instructions, precautions, and possible side effects. • 600 mg: light green, capsule shaped, film coated tablet imprinted with and 636 on one side and bisected on the other side contains 600 mg of gabapentin, USP.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |