Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
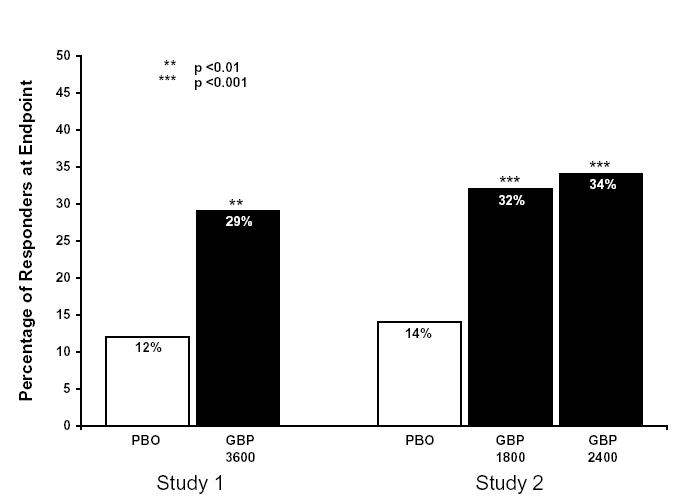
Contraindications Gabapentin Tablets, USP are contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. Standard solution Dissolve accurately weighed quantities of USP Gabapentin RS and USP Gabapentin Related Compound A RS in Diluent to obtain a solution having a known concentration of about 0.04 mg of each per mL. Test solution Remove and weigh the contents of not fewer than 20 Capsules. Gabapentin Capsules, USP 300 mg are available for oral administration as hard gelatin capsules with a white opaque body and a yellow opaque cap. “APO 113” is imprinted on each capsule in black ink; supplied in: Gabapentin USP / EP is a white or almost white, crystalline powder. It is soluble in water, in dilute acids and dilute solutions of alkali hydroxides. The starting dose is 300 mg three times a day. The recommended maintenance dose of gabapentin Capsules is 300 mg to 600 mg three times a day. Dosages up to 2400 mg/day have been administered in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration. Administer gabapentin capsules three times a day using 300 mg USP 35 Gabapentin RS, USP Gabapentin Related Compound A RS, and USP Gabapentin Related Compound B RS, respectively. Test solution—Use the Assay preparation. Standard solution—Dissolve a suitable quantity of USP Gabapentin Related Compound E RS in Diluent to obtain a solu-tion having a known concentration of 8.4 mg per mL. Chromatographic system (see Chromatography á621ñ)—Pre-pare as Gabapentin, USP is a white to off-white crystalline solid with a pKa1 of 4.72±0.10 and a pKa2 of 10.27±0.29. It is freely soluble in water and both basic and acidic aqueous solutions. Gabapentin USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as: Diluent, Buffer solution, Mobile phase, and Chromatographic system— Proceed as directed in the Assay. Impurities solution— Dissolve suitable quantities of USP Gabapentin Related Compound A RS and USP Gabapentin Related Compound B RS in methanol to obtain a solution containing about 1.4 mg per mL and 0.84 mg per mL, respectively. United States Pharmacopeia (). USP Monographs, Gabapentin Tablets. . Rockville, MD: United States Pharmacopeia. The active ingredient in gabapentin capsules is gabapentin, USP which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and a molecular Gabapentin 1 in which rU and rS are the peak responses for the Test solution and the Working standard solution, respectively; CS is the concentra-tion, in mg per mL, of the Working standard solution; 900 is the volume, in mL, of Medium; 100 is the conversion factor to percent-age; and L is the Tablet label claim, in mg. Tolerances—Not less than 80% (Q) of the labeled amount of gabapentin DEFINITION Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. United States Pharmacopeia (). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The inactive ingredients for the capsules are corn starch Horizant (gabapentin enacarbil) is an extended release tablet used to treat restless legs syndrome and for the pain from having shingles (postherpetic nerve pain). Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older Warnings Gabapentin Capsules package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) belongs to the family of medicines called antiepileptic drugs and is used for treating epilepsy (seizures). Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch, copovidone, poloxamer 407, magnesium stearate, polyethylene glycol, talc, hypromellose, titanium dioxide, macrogol, polysorbate 80 and purified water. View the USP Certificate or Product Information Sheet in the table above to view additional product details, including available label text and storage information.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |