Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
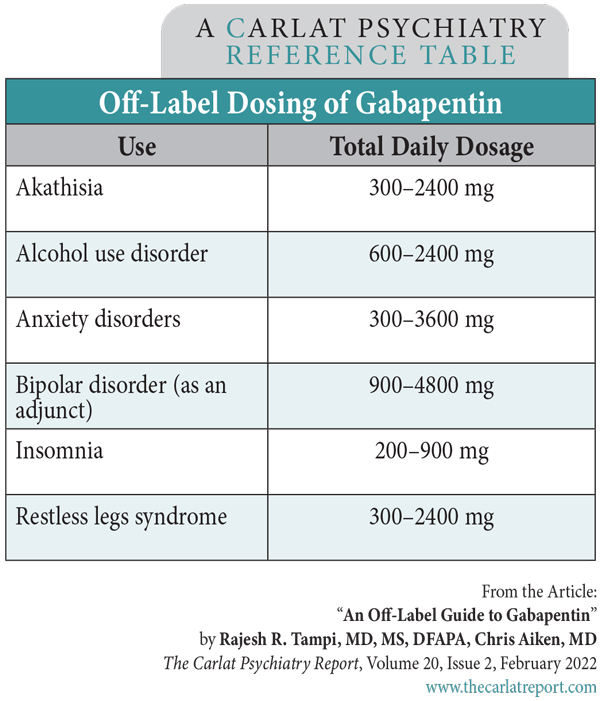
13. Sacha GL, Foreman MG, Kyllonen K, Rodriguez RJ. The use of gabapentin for pain and agitation in neonates and infants in a neonatal ICU. The Journal of Pediatric Pharmacology and Therapeutics. 2017;22(3):207-11. Gabapentin for management of neuropathic pain, irritability, neonatal abstinence syndrome, rescue sedation, feeding intolerance and visceral hyperalgesia in infants has grown over the past decade. There remains little guidance for indications, Meanwhile, the gabapentin dose was maintained at 5 mg/kg twice a day until day of life 15 when the weaning from gabapentin drug treatment was started. Gabapentin was gradually weaned to 7.5 mg twice a day (on day of life 27), followed by 5 mg twice a day (on day of life 34). When evaluated on day of life 35 her mother denied any withdrawal Gabapentin, an anticonvulsant, neuroleptic, and pain medication, is widely used in both adults and children for management of epilepsy, bipolar illness, and neuropathic pain. Gabapentin use has also been recommended for hyperemesis gravidarum and restless leg syndrome in pregnant mothers. In the pediatric population, pain is frequently under-recognized and inadequately treated. Improved education and training of health care providers can positively impact the management of pain in children. The purpose of this review is to provide a practical clinical approach to the management of acute pain in the pediatric inpatient population. Gabapentin Pediatric Medication This information from Lexicomp ® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Max dosage 3600mg if patient already on gabapentin Taper dose > 7 days to discontinue [1] Pediatric Dosing Partial seizures Adjunct for partial seizures with out secondary generalization in patients> 12yo with epilepsy; also adjunctive therapy for partial seizures in patients 3-12 years <3 years: Safety and efficacy not established We would like to show you a description here but the site won’t allow us. The primary objective was to describe gabapentin dosing, and the secondary objectives were to identify outcomes to assess efficacy and describe weaning practices. METHODS A retrospective single-center study was performed in infants younger than 1 year who received gabapentin at Boston Children’s Hospital between 2015 and 2021. Background: Opioid and sedative medications are commonly used to treat pediatric patients with congenital heart disease; however, their use is not without adverse effects. Symptoms of withdrawal can occur if the medications are discontinued abruptly or weaned too quickly. Objective: The aim of this study was to understand and describe the current management of opioid and sedative weaning in Gabapentin has shown benefits for a variety of pain etiologies in adult patients, with off-label use as an adjunctive agent in pediatric patients occurring more frequently. METHODS We performed a retrospective chart review of infants receiving gabapentin for NOWS. Data points collected included patient’s sex, gestational age, maternal opioid exposure, NOWS medication dosing and length of therapy, number of failed wean attempts, time to successful morphine wean and duration of morphine wean, length of stay in the neonatal intensive care unit (NICU), and NOWS PURPOSE: Gabapentin, a gamma-aminobutyric acid (GABA) analog with antiepileptic and antinociceptive properties, is increasingly reported in the literature for the treatment of pain and agitation in medically complex patients in the neonatal intensive care unit (NICU), despite the paucity of safety and efficacy data in infants. The objectives of this study were to characterize gabapentin Several pediatric case reports and case series have described the use of gabapentin in children with neuropathic pain.11-14 In 1998, McGraw and Brett successfully used gabapentin in a 12 year old girl with post-thoracotomy pain of 3 months’ duration.11 Numerous other therapies, including oral opioids, benzodiazepines, and non-steroidal anti PICU Clinical Pathway for Sedation/AnalgesiaWeaning Quick Links Converting IV to Enteral Sedation Calculating the Dose Opioid Benzodiazepine Steps in Transitioning from Continuous IV Sedation to Intermittent Enteral Regimen Weaning Enteral Sedation Duration 5-9 Days or High Risk < 5 Days – PO Duration 10-21 Days or High Risk 5-9 Days – PO Duration > 21 Days or High Risk 10-21 Days – PO Gabapentin has been used in the management of pediatric neuropathic pain for nearly two decades. In 1998, McGraw and Stacey described the effective use of gabapentin in a 12-year-old girl with neuropathic pain after thoracotomy for a pacemaker revision.12 She described her pain as knife-like and almost constant. The decision to begin gabapentin therapy depended on admitted prenatal use, a failure to wean or escalation of methadone, and sustained behavioral changes. All these infants were weaned to methadone 0.02 mg every 8 hours (step 6), followed by gabapentin weaning by 25% every 4 days coordinated with methadone weaning every 48 hours. Clinicians should be aware of possible withdrawal symptoms from drugs such as gabapentin, administered to mothers during pregnancy. We also encourage the gradual tapering of gabapentin in neonates over weeks to months similar to the adult population. Gabapentin was well tolerated in infants. Initial gabapentin dosing of 5 mg/kg/dose every 24 hours appears safe and consistent with other published studies in infants. The improvement in outcomes with few adverse events suggests a beneficial role for gabapentin. Heterogeneity of pain type and gabapentin dosing regimens within the included studies made conclusions difficult to quantify. Efficacy likely depends significantly on etiology of pain; however, per these studies, gabapentin is likely safe to use for a variety of pediatric patient populations as a mu
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |