Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 | |
 |  |
 |  |
 |  |
 |  |
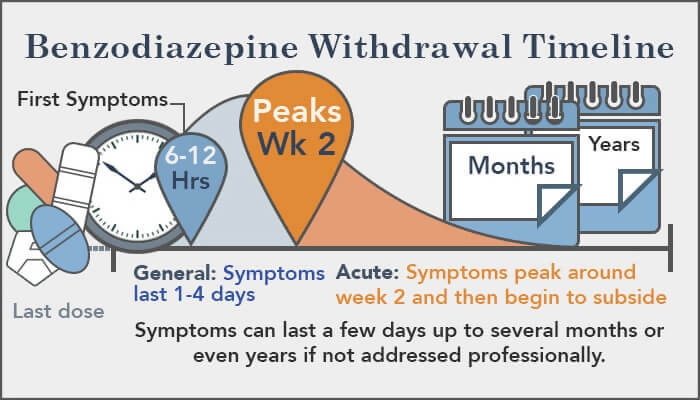
We describe a single center experience with gabapentin as adjunctive therapy in infants with neonatal opioid withdrawal syndrome (NOWS). We performed a retrospective chart review of infants receiving gabapentin for NOWS. Gabapentin, an anticonvulsant, neuroleptic, and pain medication, is widely used in both adults and children for management of epilepsy, bipolar illness, and neuropathic pain. Gabapentin use has also been recommended for hyperemesis gravidarum and restless leg syndrome in pregnant mothers. Introduction Gabapentin is a gamma-aminobutyric acid analog that has been used in multiple disease states in children, including neuropathic pain, irritability, visceral hyperalgesia, neonatal abstinence syndrome (NAS), rescue sedation and feeding intolerance. 1 – 7 Despite the increased utilization of gabapentin in neonates, 1 there remains a gap in the pediatric literature describing the We present a newborn who showed signs of withdrawal after prolonged in utero exposure to gabapentin. Clinicians should be aware of possible withdrawal symptoms from drugs such as gabapentin, administered to mothers during pregnancy. OBJECTIVE: Although gabapentin use is deemed safe during pregnancy, no clinical reports of gabapentin withdrawal syndrome in a neonate have been described. RESULTS: We present a newborn who showed signs of withdrawal after prolonged in utero exposure to gabapentin. Among the documented cases, gabapentin withdrawal began between 12 hours and 7 days after the last dose. The majority saw withdrawal symptoms within 24 to 48 hours. Among the cases reported, gabapentin withdrawal symptoms typically peaked three days after someone’s last dose. Gabapentin has been used to treat neuropathic pain related to visceral hyperalgesia in the neonatal population.5,6 Gabapentin, a gamma-aminobutyric acid analog, is thought to inhibit pain via voltage-dependent calcium ion channels in the central nervous system. There is minimal information on the use and side effects of initiating and discontinuing of gabapentin in this population.3,5-7 The CONCLUSION The majority received gabapentin at a median dose of 14 mg/kg/day as Step 1 for delirium. Gabapentin was associated with a significant decrease in pain and withdrawal scores. We report a retrospective case series of 19 infants exposed to both opioids and gabapentin prenatally. We describe a unique behavioral phenotype in 15 of these infants and report a treatment strategy. This large population based cohort study showed a 30-60% increase in the risk of neonatal drug withdrawal associated with co-exposure to antidepressants, benzodiazepines, and gabapentin compared with opioids alone; no significant increase in risk was observed for atypical antipsychotics and Z drugs (non-benzodiazepine hypnotics) We present a newborn who showed signs of withdrawal after prolonged in utero exposure to gabapentin. Clinicians should be aware of possible withdrawal symptoms from drugs such as gabapentin, administered to mothers during pregnancy. Adverse events were reported in five of the 11 patients, and three were related to abrupt discontinuation of gabapentin due to NPO status, suggesting the existence of a gabapentin withdrawal syndrome in infants. Despite some clinician advocacy for the use of gabapentin to treat neonatal irritability of presumed neurologic origin, the extent of gabapentin administration to hospitalized neonates is unknown. We aimed to identify trends in gabapentin Despite some clinician advocacy for the use of gabapentin to treat neonatal irritability of presumed neurological origin, the extent of gabapentin administration to hospitalized neonates is unknown. We aimed to identify trends in gabapentin utilization among infants hospitalized in neonatal intensive care units (NICUs) across the United States and to evaluate the associations between clinical Gabapentin for management of neuropathic pain, irritability, neonatal abstinence syndrome, rescue sedation, feeding intolerance and visceral hyperalgesia in infants has grown over the past decade. There remains little guidance for indications, Results: We present a newborn who showed signs of withdrawal after prolonged in utero exposure to gabapentin. Clinical implications: Clinicians should be aware of possible withdrawal symptoms from drugs such as gabapentin, administered to mothers during pregnancy. Results The absolute risk for neonatal drug withdrawal ranged from 1.0% in infants exposed in utero to prescription opioids alone to 11.4% for those exposed to opioids co-prescribed with gabapentin. We would like to show you a description here but the site won’t allow us. One study found that when gabapentin is combined with opioids late in pregnancy, withdrawal can occur. It is not known how often withdrawal occurs in babies exposed to this combination. Withdrawal symptoms were also reported in infants born to women treated with gabapentin or baclofen during pregnancy for the management of neuropathic pain and paraplegia, respectively [174,175, 179].
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 | |
 |  |
 |  |
 |  |
 |  |