Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
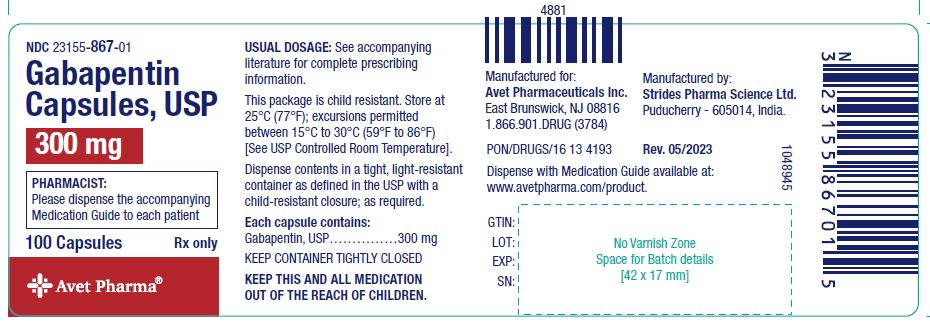
Frequently Asked Questions What is NDC 59762-5050-7? The NDC Packaged Code 59762-5050-7 is assigned to a package of 1 bottle, plastic in 1 carton / 470 ml in 1 bottle, plastic of Gabapentin, a human prescription drug labeled by Mylan Pharmaceuticals Inc.. The product's dosage form is suspension and is administered via oral form. The active ingredient in Gabapentin Oral Solution is gabapentin,which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C - 9H Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Legacy Greenstone productsGreenstone Product Chart Active ingredient: gabapentin 250 mg/5 mL The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Gabapentin requires a prescription from your veterinarian. This medication treats chronic pain such as pain associated with arthritis and other joint problems. In addition, it can be used to treat some seizure disorders. While the mechanism of action for this medication is unknown, it is believed that gabapentin works by decreasing calcium influx and inhibiting excitatory neurotransmitters DESCRIPTION Neurontin® (gabapentin) capsules, Neurontin® (gabapentin) tablets, and Neurontin® (gabapentin) oral solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Neurontin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor. All the best, Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. The active ingredient in gabapentin oral solution is gabapentin, which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: artificial cool strawberry anise flavor, glycerin, purified water, and xylitol. NDC 59762-5025 GABAPENTIN Gabapentin GABAPENTIN is a Oral Suspension in the Human Prescription Drug category. It is labeled and distributed by Greenstone Llc. The primary component is Gabapentin. Gabapentin is a human prescription drug by Mylan Pharmaceuticals Inc.. The product is distributed in a single package with NDC code 59762-5050-7.Gabapentin is DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. DESCRIPTION Gabapentin Oral Solution is supplied as oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients are anise flavor natural and artificial, cooling agent flavor artificial, glycerin, purified water, strawberry flavor artificial and xylitol. Gabapentin is described as 1- (aminomethyl) cyclohexaneacetic acid with a molecular formula of C 9 H 17 NO 2 and a molecular Coupler LLC: Gabapentin tablets are indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Gabapentin is available as oral capsules of 100 mg, 300 mg, and 400 mg; tablets of 100 mg, 300 mg, 400 mg, 600 mg, and 800 mg; and an oral solution of 50 mg/mL. However, the need often exists for a higher-concentration oral liquid, and if tablets or capsules are used as the drug source, a suspension will result. DESCRIPTION Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |