Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 | 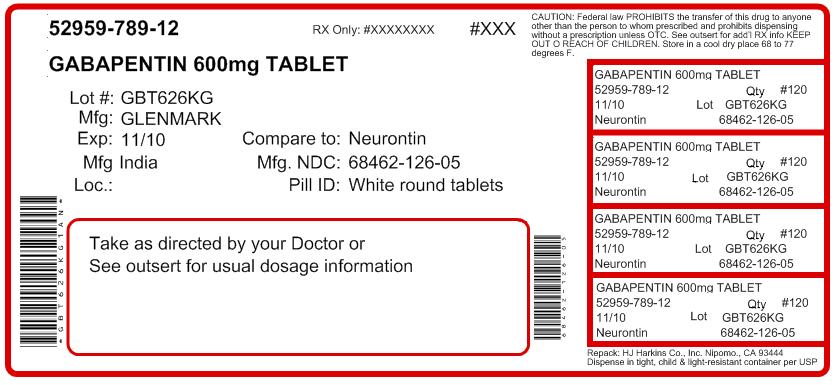 |
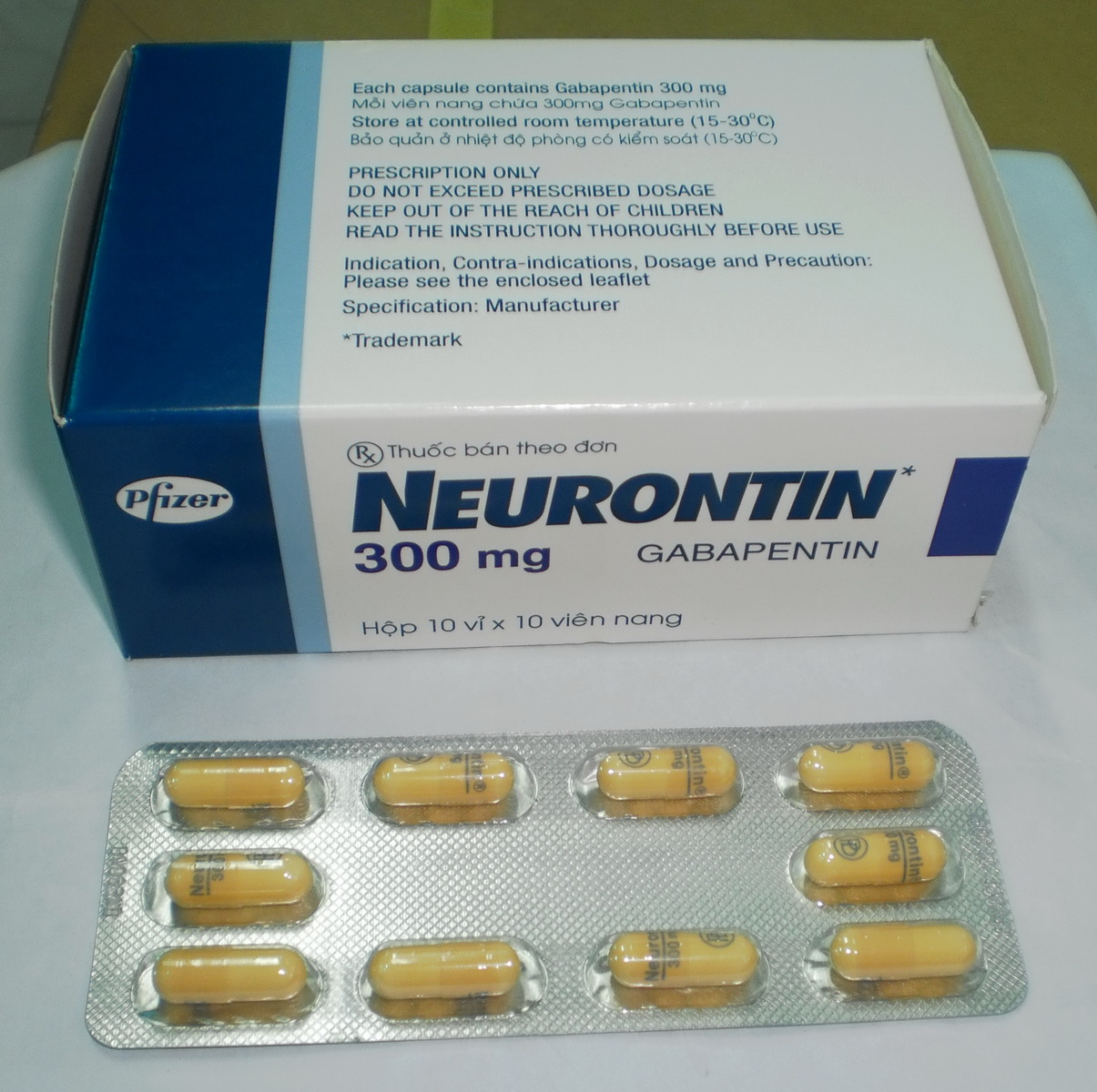
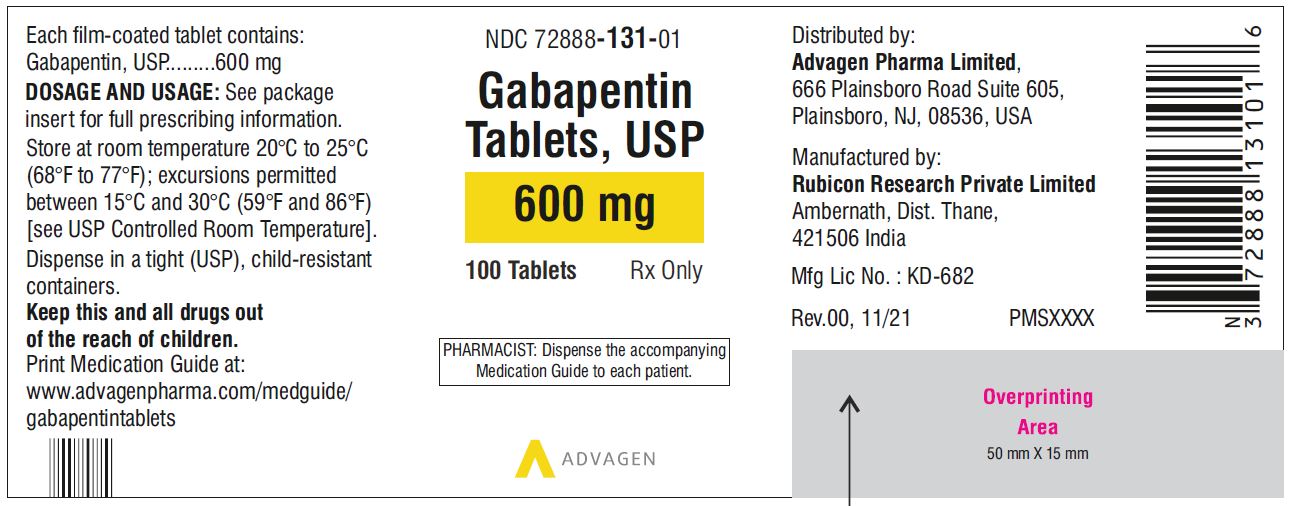
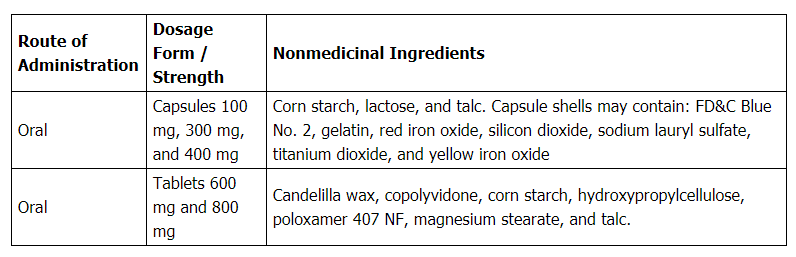
Each gabapentin tablet, USP intended for oral administration contains 600 mg and 800 mg of gabapentin. In addition each tablet contains following inactive ingredients: copovidone, low substituted hydroxypropyl cellulose, magnesium stearate, mannitol, poloxamer, povidone and talc. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The inactive ingredients for the capsules are corn starch DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. 2 CONTRAINDICATIONS TEVA-GABAPENTIN is contraindicated in patients who are hypersensitive to this drug or to any ingredient in the formulation, including any non-medicinal ingredients, or component of the container. For a complete listing, see 6 DOSAGE FORMS, STRENGTHS, COMPOSITION AND PACKAGING. Do not take gabapentin capsules if you are allergic to gabapentin or any of the other ingredients in gabapentin capsules. See the end of this Medication Guide for a complete list of ingredients in gabapentin capsules. Gabapentin tablets are contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity - Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The active ingredient in NEURONTIN capsules, tablets, and oral solution is gabapentin,which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the molecular weight is 171.24. Gabapentin is a white to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. Each NEURONTIN tablet contains 600 mg or 800 mg of gabapentin and the following inactive ingredients: poloxamer 407, copovidone, cornstarch, magnesium stearate, hydroxypropyl cellulose, talc, and candelilla wax Gabapentin Tablets, USP are contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity - Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan Do not take gabapentin tablets if you are allergic to gabapentin or any of the other ingredients in gabapentin tablets. See the end of this Medication Guide for a complete list of ingredients in gabapentin tablets. Gabapentin, a medication widely used for treating nerve pain and certain types of seizures, has gained considerable attention in both medical and non-medical contexts. Understanding what goes into this drug can provide insights into its effectiveness, potential side effects, and overall safety. The composition of gabapentin is essential for both healthcare providers and patients to know, as it Gabapentin tablet is contraindicated in patients with demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS Once-daily gabapentin tablets are not substitutable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration. The safety and Gabapentin Tablets package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin tablets are contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has occurred with Do not take gabapentin if you are allergic to gabapentin or any of the other ingredients in gabapentin. See the end of this Medication Guide for a complete list of ingredients in gabapentin. Each NEURONTIN tablet contains 600 mg or 800 mg of gabapentin and the following inactive ingredients: poloxamer 407, copovidone, cornstarch, magnesium stearate, hydroxypropyl cellulose, talc, and candelilla wax MEDICATION GUIDE GABAPENTIN CAPSULE, USP GABAPENTIN TABLETS, USP (GAB-a-PEN-tin) What is the most important information I should know about gabapentin? Do not stop taking gabapentin without first talking to your healthcare provider. Stopping gabapentin suddenly can cause serious problems. Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |