Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
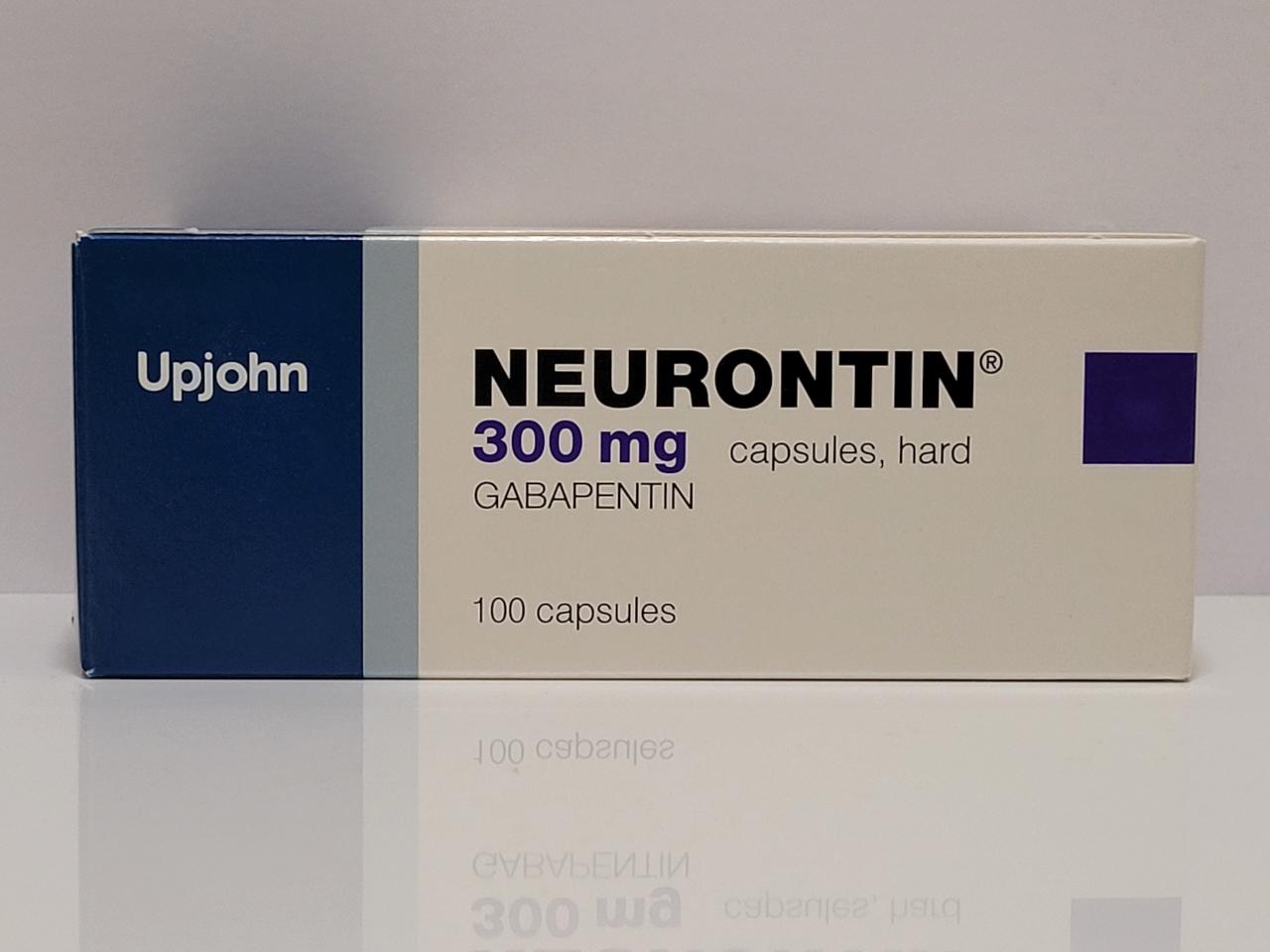 |  |
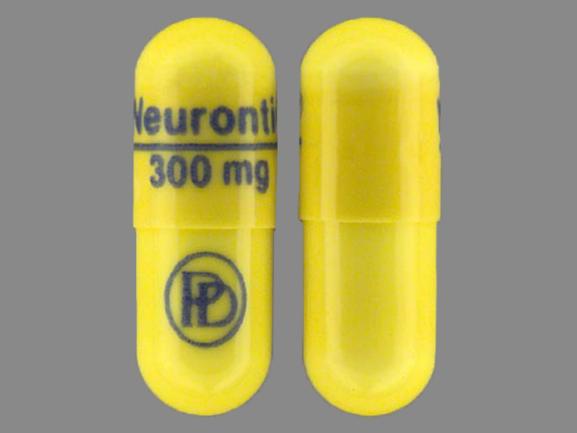
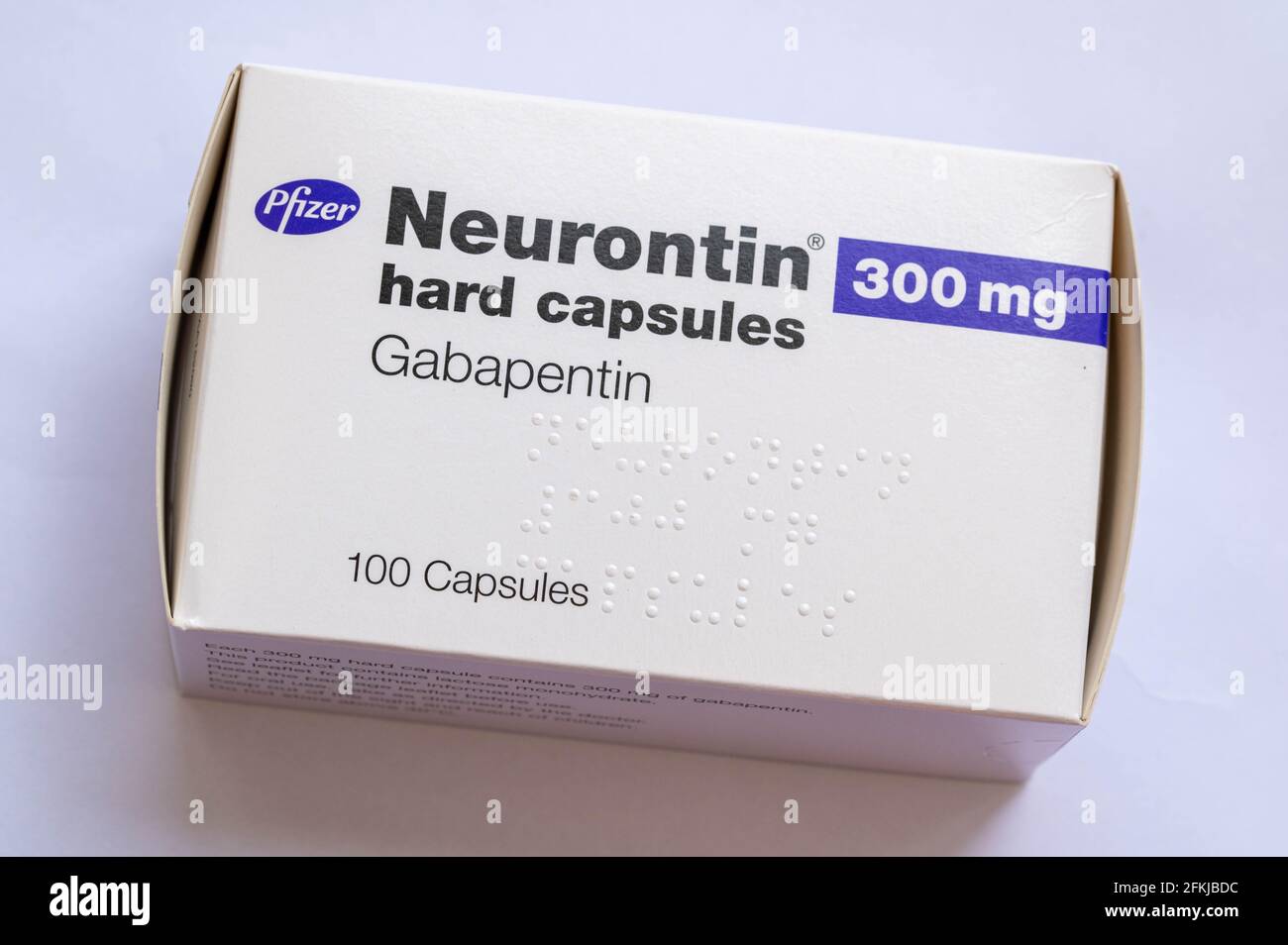
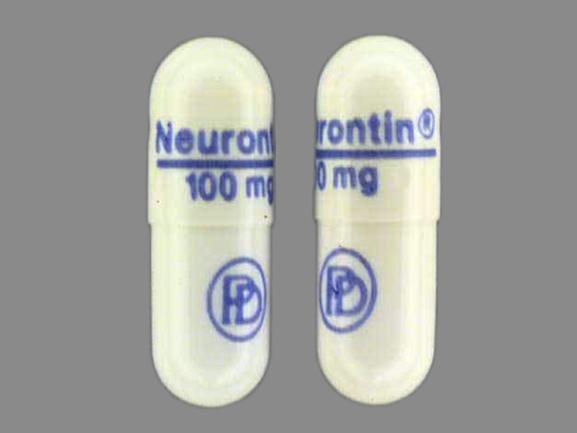
DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrNEURONTIN® Gabapentin Capsules Capsules, 100 mg, 300 mg and 400 mg, oral Do not take NEURONTIN if you are allergic to gabapentin or any of the other ingredients in NEURONTIN. See the end of this Medication Guide for a complete list of ingredients in NEURONTIN. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The inactive ingredients for the capsules are corn starch 2.1 Dosage for Postherpetic Neuralgia In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In clinical studies, efficacy was Neurontin is an analgesic and antiepileptic medication used in the treatment of postherpetic pain and as an adjunctive therapy for partial seizures. Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. The active ingredient in NEURONTIN capsules, tablets, and oral solution is gabapentin, which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is Each Neurontin capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Each capsule for oral administration contains 100 mg, 300 mg and 400 mg of gabapentin. In addition, each capsule contains the following inactive ingredients: croscarmellose sodium and magnesium stearate. The 100 mg, 300 mg and 400 mg capsule imprinting ink contains the following inactive ingredients: ammonium hydroxide; black iron oxide, bacteria controlled EEC No. 172; n-butyl; ethyl alcohol DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). 2.1 Dosage for Postherpetic Neuralgia - In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and Neurontin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). MEDICATION GUIDE GABAPENTIN CAPSULES, USP (gab′′ a pen′ tin) 100 mg, 300 mg and 400 mg Read the Medication Guide before you start taking gabapentin capsules and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. 300 mg Each hard gelatin Coni-Snap capsule with yellow opaque body and cap printed with "PD" on one side and "Neurontin/300 mg" on the other contains 300 mg of gabapentin. The 300 mg and 400 mg capsule shell contains FD&C Red 40, D&C Yellow 10, titanium dioxide, gelatin and sodium lauryl sulfate. The ink ingredients common for all strengths are shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide and potassium hydroxide. Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |