Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
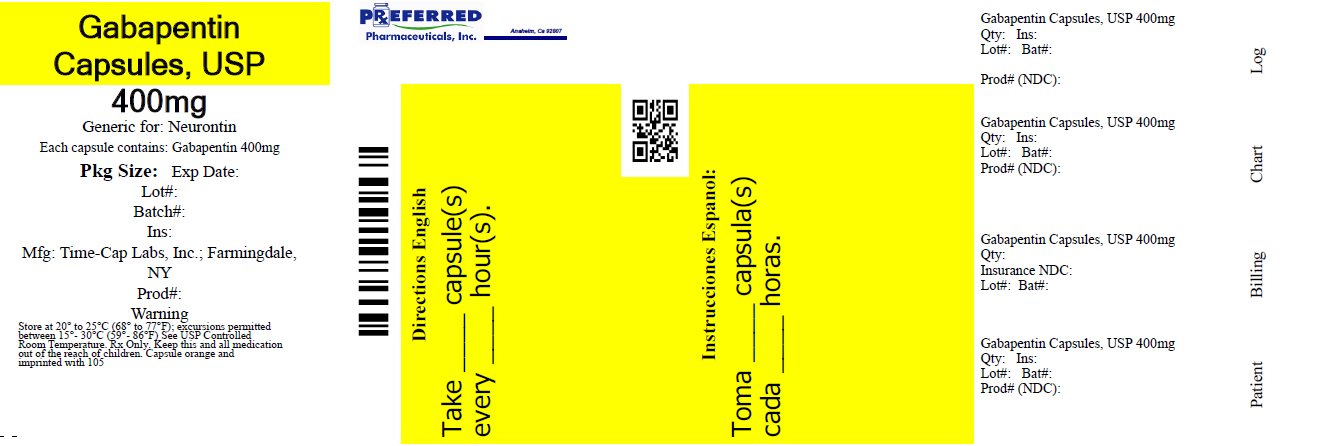
The active ingredient in Gabapentin Capsules USP is gabapentin, which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the molecular TEVA-GABAPENTIN Capsules: Nonmedicinal ingredients include colloidal silicon dioxide, lactose monohydrate, pregelatinized starch, and talc. Capsule shells may contain gelatin, titanium dioxide, yellow iron oxide (300 mg and 400mg), red iron oxide (400 mg), and Blue ink. DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. Save money on your Neurontin® Capsules prescription by switching to Teva's FDA-approved generic version, Gabapentin Capsules, USP DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The recommended maintenance dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. Gabapentin Overview The Patient Information Leaflet for products containing Gabapentin from Teva can be downloaded below, along with other information such as Frequently Asked Questions where applicable. To contact Teva's Medical Information Service email: medinfo@tevauk.com or telephone: 0207 540 7117. NDC 0093-4444 Gabapentin Gabapentin Gabapentin is a Oral Tablet in the Human Prescription Drug category. It is labeled and distributed by Teva Pharmaceuticals Usa, Inc.. The primary component is Gabapentin. Who should not take gabapentin capsules? Do not take gabapentin capsules if you are allergic to gabapentin or any of the other ingredients in gabapentin capsules. See the end of this Medication Guide for a complete list of ingredients in gabapentin capsules. What should I tell my healthcare provider before taking gabapentin capsules? Teva-Gabapentin: La gabapentine appartient à la classe des médicaments appelés antiépileptiques. Elle s'utilise en association avec d'autres médicaments de même nature pour le traitement et la prévention des crises d'épilepsie. La gabapentine ne guérit pas l'épilepsie; elle maîtrise les crises seulement pendant les périodes où le médicament est pris. Le médicament agit en What you need to know before you take Gabapentin Teva Do not take Gabapentin Teva: if you are al ergic (hypersensitive) to gabapentin or any of the other ingredients of this medicine (listed in section 6). arnings and precautions Talk to your doctor or pharmacist before taking Gabapentin Teva if suffer from kidney problems your doctor may TEVA-GABAPENTIN Tablets: Nonmedicinal ingredients include povidone, microcrystalline cellulose, crospovidone, talc, hydrogenated vegetable oil and film coating: polyvinyl alcohol, titanium dioxide, macrogol and talc. Teva-Gabapentin: Gabapentin belongs to the class of medications called anti-epileptics. It is used in combination with other seizure control medications to manage and prevent seizures associated with epilepsy. Gabapentin does not cure epilepsy and only works to control seizures as long as the medication is taken. Gabapentin works by affecting the transmission of nerve signals in the brain. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The inactive ingredients for the capsules are corn starch Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. TEVA-GABAPENTIN is contraindicated in patients who are hypersensitive to this drug or to any ingredient in the formulation, including any non-medicinal ingredients, or component of the container. Patients taking NEURONTIN should not drive until they have gained sufficient experience to assess whether NEURONTIN impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. The ink ingredients common for all strengths are shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide and potassium hydroxide. Summary of medicine characteristics contains detail information about dosage for adults and children , composition and side effects of medicine GABAPENTIN TEVA 300 MG CAPSULES HARD Dosage: Capsule, Strength: 300 mg, Brand name equivalent: Generic of Neurontin® Capsules
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |