Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 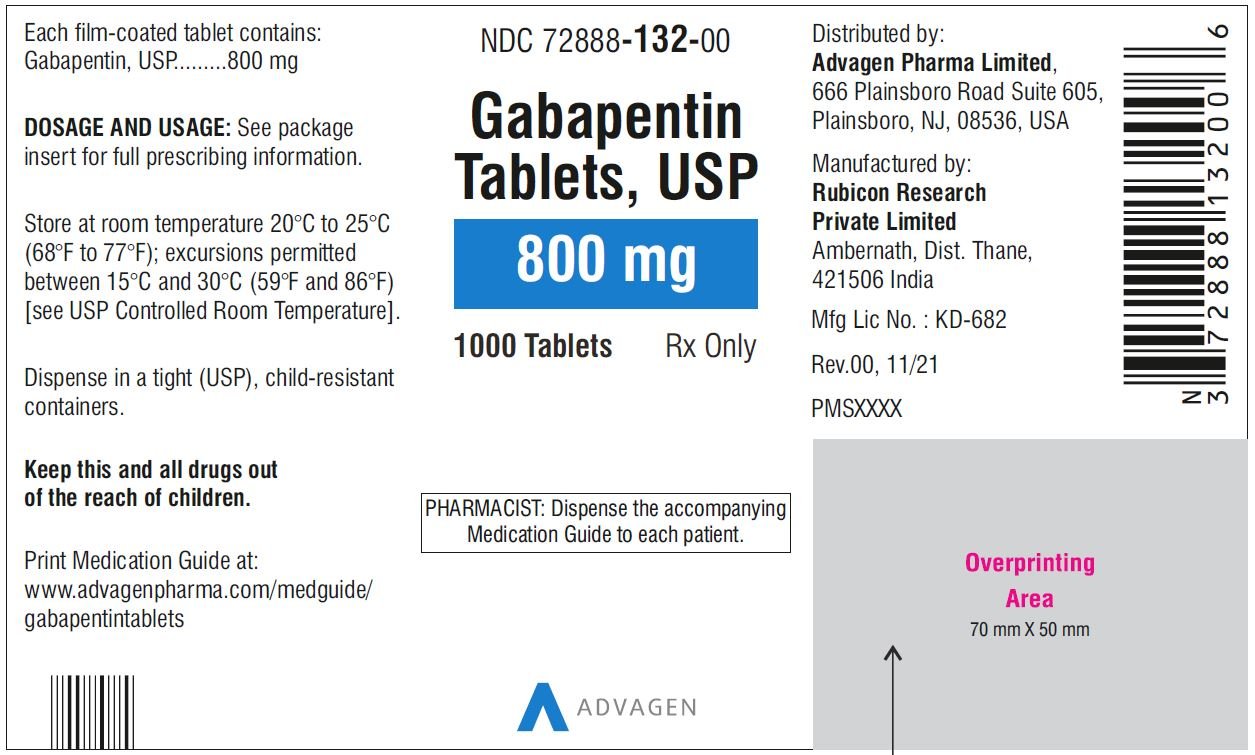 |
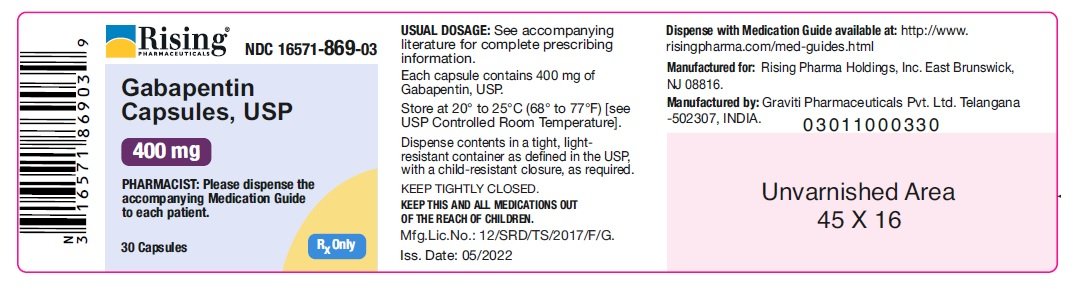
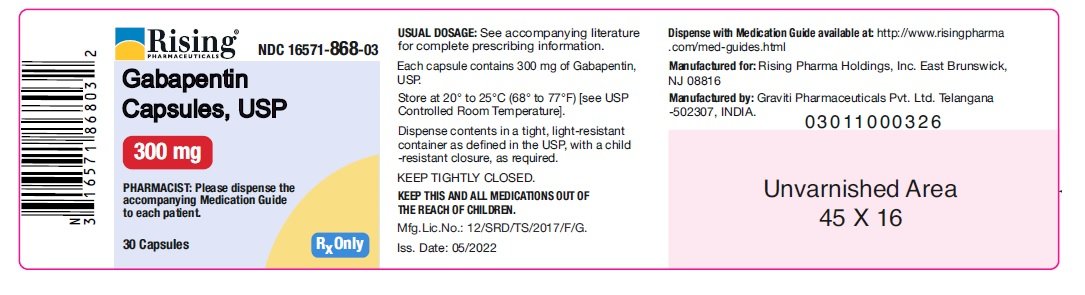
Neurontin (gabapentin) is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age with epilepsy. A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. For more information about Gabapentin Oral Solution, medical inquiries, or to report side effects regarding Gabapentin Oral Solution, please call 1-800-541-4802. Order Gabapentin 250 mg / 5 mL Solution 470 mL by Acella Pharmaceuticals 42192060816 Gabapentin is a human prescription drug by Acella Pharmaceuticals, Llc. The product is distributed in 5 packages with NDC codes 42192-608-05, 42192-608-06, PRINCIPAL DISPLAY PANEL - Gabapentin Oral Solution 5 mL Unit Dose Cup. ALWAYS DISPENSE WITH MEDICATION GUIDE - Acella Pharmaceuticals, LLC - 5 mL unit dose cup: NDC 42192-608-05 - Gabapentin Oral Solution - 250 mg per 5 mL - 5 mL unit dose cups, boxes of 40 PRINCIPAL DISPLAY PANEL - Gabapentin Oral Solution 6 mL Unit Dose Cup. king gabapentin. What is Gabapent. e used to treat: • Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infe. tion) in adults. • Partial seizures when taken together with other medicines in adults and children 3 years of age and old. See full prescribing information for GABAPENTIN ORAL SOLUTION. Patients 12 years of age and older: starting dose is 300 mg three times daily; may be titrated up to 600 mg three times daily. From patients with hypothyroidism, acne, eczema, cystic acne, UTIs, low iron, IBS, nutritional deficiencies, arthritis, coughs and colds to expecting mothers in need of prenatal care, our team of experts work hard to deliver on products that help patients make their life a little better! Page 7: Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in DESCRIPTION Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The NDC Packaged Code 42192-608-16 is assigned to a package of 470 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Acella Pharmaceuticals, Llc. Gabapentin Oral Solution is indicated for: •Epilepsy with Partial Onset Seizures ( 2.2) •Patients 12 years of age and older: starting dose is 300 mg three times daily; may be titrated up to 600 mg three times daily. Page 2: Acella Pharmaceuticals, LLC: Gabapentin Oral Solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Page 8: Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in The effective dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. NDC 42192-608 Gabapentin Gabapentin Gabapentin is a Oral Solution in the Human Prescription Drug category. It is labeled and distributed by Acella Pharmaceuticals, Llc. The primary component is Gabapentin. Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in Please see links below for Full Prescribing Information, including BOXED WARNING and Important Safety Information. To report suspected adverse reactions, contact the FDA at (800) FDA-1088 or www.fda.gov/medwatch. Acella Pharmaceuticals, LLC: Gabapentin Oral Solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |