Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
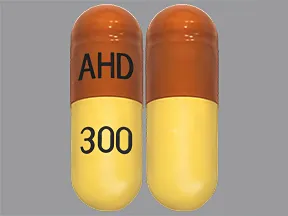
Complete details for NDC 65162-0102-11 Gabapentin 300 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000130 The NDC Packaged Code 69097-814-12 is assigned to a package of 500 capsule in 1 bottle of Gabapentin, labeled by Cipla Usa Inc.. The product's dosage form is and is administered via form. The NDC code 45963-556 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Actavis Pharma, Inc.. The product's dosage form is capsule and is administered via oral form. The product is distributed in 4 packages with assigned NDC codes 45963-556-00 10000 capsule in 1 box , 45963-556-11 100 capsule in 1 bottle , 45963-556-50 500 capsule in 1 300 mg Capsules (Yellow/Yellow colored, size '1' hard gelatin capsules with "104" printed on body of capsules containing white to off white granular powder) NDC - 60760-088-30 BOTTLES OF Complete details for NDC 49483-0606-50 GABAPENTIN 300 mg/1 including product information, packaging information, pricing, prescribing information and package photos. Order Gabapentin 300 mg Capsule 100 Capsules by NorthStar Rx 16714066201 Manufacturers of Gabapentin that have been granted an NDC (National Drug Code). Gabapentin records from the RxChat NDC Database1,246 records found Page:1 Start a Discussion 0093-4443 Jul 11, 2008Gabapentin 600 mg Oral Tablet by Teva Pharmaceuticals USA Inc 0093-4444 Aug 01, 2008Gabapentin 800 mg Oral Tablet by Teva Pharmaceuticals USA Inc 0143-9994 Jul 09, 2007Gabapentin 400 mg Oral Capsule by West-ward Pharmaceutical Corp 0228-2636 Dec 14, 2004Gabapentin 600 mg Oral SPL UNCLASSIFIED SPL MEDGUIDE PACKAGE LABEL.PRINCIPAL DISPLAY PANEL NDC 70771-1861-9 in bottle of 90 tablets - Gabapentin tablets, 300 mg - Rx only - 90 tablets - gabapentin 300 mg - NDC 70771-1862-9 in bottle of 90 tablets - Gabapentin tablets, 600 mg - Rx only - 90 INGREDIENTS AND APPEARANCE Complete details for NDC 49483-0606-10 GABAPENTIN 300 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000130 Dosage: Capsule, Strength: 300 mg, Brand name equivalent: Generic of Neurontin® Capsules The NDC code 63739-903 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Mckesson Corporation Dba Sky Packaging. The product's dosage form is capsule and is administered via oral form. The product is distributed in a single package with assigned NDC code 63739-903-10 10 blister pack in 1 carton / 10 capsule in 1 blister pack. This page This web page contains comprehensive information about NDC Code 63739-903-10. “Gabapentin ” (aka “Gabapentin”) is a human prescription drug product labeled by “McKesson Corporation dba Sky Packaging”. 2.1 Dosage for Postherpetic Neuralgia - In adults with postherpetic neuralgia, gabapentin capsules may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a NDC 69097-943 GABAPENTIN Gabapentin GABAPENTIN is a Oral Capsule in the Human Prescription Drug category. It is labeled and distributed by Cipla Usa Inc.. The primary component is Gabapentin. Complete details for NDC 69097-0943-12 GABAPENTIN 300 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000130 The NDC Packaged Code 45963-556-50 is assigned to a package of 500 capsule in 1 bottle of Gabapentin, a human prescription drug labeled by Actavis Pharma, Inc.. The NDC code 65162-102 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Amneal Pharmaceuticals Llc. The product's dosage form is capsule and is administered via oral form. Gabapentin List of products in the National Drug Code with proprietary name gabapentin. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. Gabapentin is known as an anticonvulsant or antiepileptic drug. The NDC Packaged Code 67877-223-05 is assigned to a package of 500 capsule in 1 bottle of Gabapentin, a human prescription drug labeled by Ascend Laboratories, Llc.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |