Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
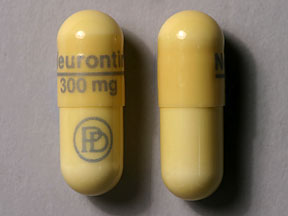
Get detailed information about the safety data sheet (MSDS) for gabapentin capsules, including its uses, side effects, precautions, and storage instructions. Material Name: Gabapentin Capsules (100 mg, 300 mg and 400 mg) Trade Name: NEURONTIN; NEUGABA; GANTIN; PARKETIN; GABAPENTIN PFIZER Chemical Family: Mixture Relevant Identified Uses of the Substance or Mixture and Uses Advised Against Intended Use: Pharmaceutical product used as anticonvulsant Generic Name: Gabapentin Capsules, USP 100, 300 and 400 mg. EMERGENCY OVERVIEW Caution Statement: Each Gabapentin Capsules intended for oral administration contains Gabapentin, USP and excipients generally considered to be non-toxic and non-hazardous in small quantities and under conditions of normal occupational exposure. Caution Statement: Each Gabapentin Tablets intended for oral administration contains Gabapentin and excipients generally considered to be non-toxic and non-hazardous in small quantities and under conditions of normal occupational exposure. We would like to show you a description here but the site won’t allow us. Canada - WHMIS: Classifications WHMIS hazard class: None required This product has been classified in accordance with the hazard criteria of the CPR and the MSDS contains all of the information required by the CPR. Gabapentin Standard for the Uniform Scheduling for Drugs and Poisons: Caution Statement: Each Gabapentin Capsules intended for oral administration contains Gabapentin, USP and excipients generally considered to be non-toxic and non-hazardous in small quantities and under conditions of normal occupational exposure. Material Name: Gabapentin Capsules (100 mg, 300 mg and 400 mg) Trade Name: Chemical Family: Intended Use: Neurontin® Mixture Pharmaceutical product used as anticonvulsant The precise mechanisms by which gabapentin produces its analgesic and antiepileptic actions are unknown. In animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). Gabapentin prevents pain-related responses in several models of neuropathic pain in rats and mice (e EMERGENCY OVERVIEW Caution Statement: Each Gabapentin Capsules intended for oral administration contains Gabapentin, USP and excipients generally considered to be non-toxic and non-hazardous in small quantities and under conditions of normal occupational exposure. 9.2. Other information No information available Capsules and Tablets No information available Trade name: Gabapentin Synonym CI-945 1-(aminomethyl)-cyclohexaneacetic acid Article number: 10008346 CAS Number: 60142-96-3 EC number: 262-076-3 Application of the substance / the mixture This product is for research use - Not for human or veterinary diagnostic or therapeutic use. Details of the supplier of the safety data sheet Manufacturer Revision note: New issue The information and recommendations in this safety data sheet are, to the best of our knowledge, accurate as of the date of issue. Nothing herein shall be deemed to create any warranty, express or implied. It is the responsibility of the user to determine the applicability of this information and the suitability of the material or product for any particular purpose. Disclaimer: The information in this MSDS is only applicable to the specified product, unless otherwise specified, it is not applicable to the mixture of this product and other substances. This MSDS only provides information on the safety of the product for those who have received the appropriate professional training for the user of the product. 300 mg Capsules: Hard Gelatin Capsules Size “1” with Yellow Opaque Cap and Yellow Opaque body imprinted with 300 mg and IG322 filled with white to Off-white powder. Material Name: Gabapentin Capsules (100 mg, 300 mg and 400 mg) Trade Name: Chemical Family: Intended Use: Neurontin® Mixture Pharmaceutical product used as anticonvulsant Disclaimer The information provided in this Safety Data Sheet is correct to the best of our knowledge, information and belief at the date of its publication. The information given is designed only as a guidance for safe handling, use, processing, storage, transportation, disposal and release and is not to be considered a warranty or quality specification. The information relates only to the SAFETY DATA SHEET Date: 08/14/2024 Generic Name: Gabapentin Capsules, USP 100, 300 and 400 mg.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |