Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
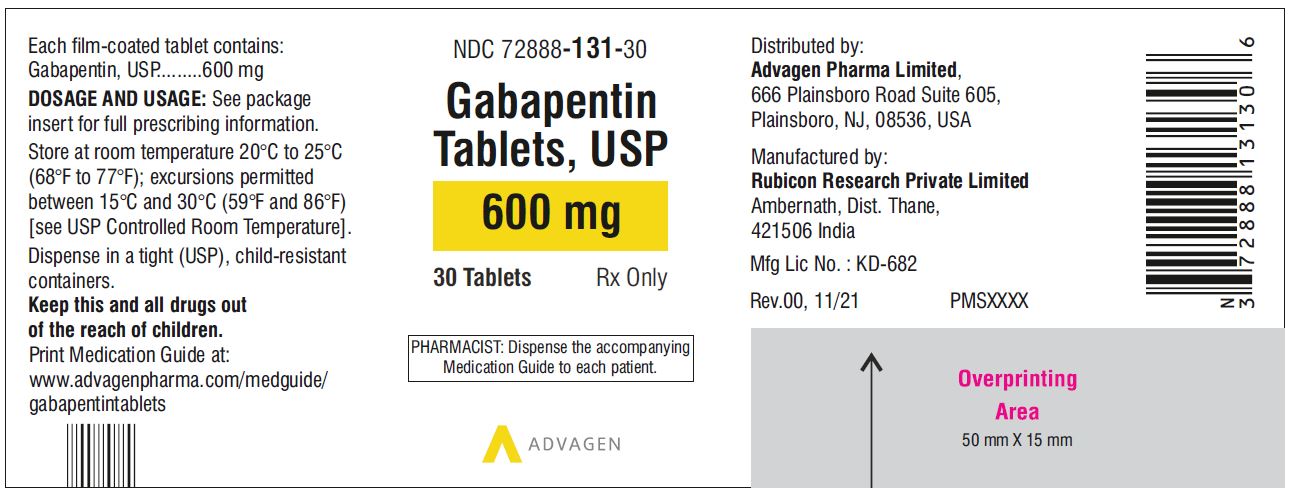
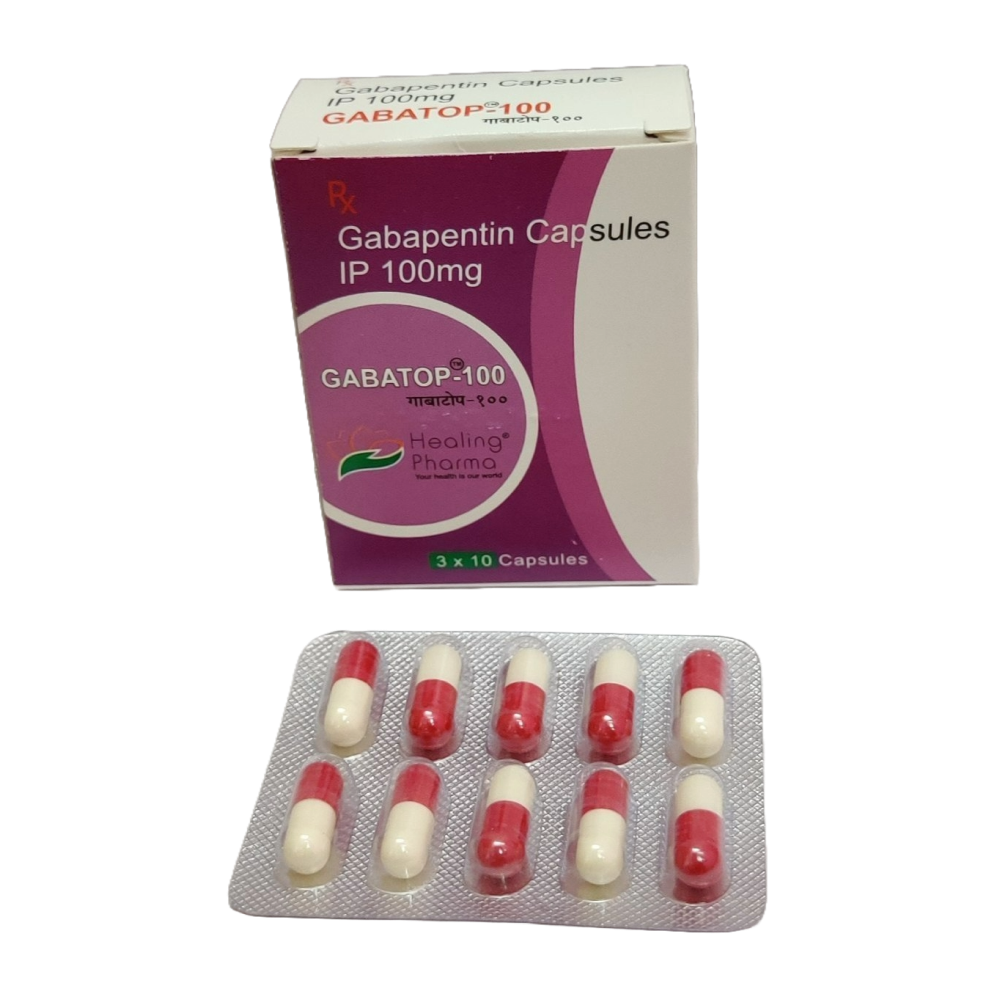
Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. Gabapentin binding at a2d subunit of voltage-activated calcium channels can increase the release of excitatory neurotransmitters and may directly or indirectly effect the dopaminergic reward system associated with addiction. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. Regulating controlled substances and precursors Examples of controlled substances and precursors include substances explicitly listed in the Controlled Drugs and Substances Act (CDSA) such as: cocaine fentanyl morphine methamphetamine ephedrine Many other substances are also captured under the CDSA as analogues, derivatives, isomers and salts of listed substances. Health Canada provides a Gabapentin belongs to the family of medicines called antiepileptic drugs and is used for treating epilepsy (seizures). While reviewing information provided by the manufacturer, Health Canada found cases of serious breathing problems in patients treated with gabapentin. Health Canada's review concluded that there is evidence supporting a risk of serious breathing problems when gabapentin is Gabapentin belongs to the family of medicines called antiepileptic drugs and is used for treating epilepsy (seizures). While reviewing information provided by the manufacturer, Health Canada found cases of serious breathing problems in patients treated with gabapentin. Briefing Note From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedati September 17, 2019 For Immediate Release OTTAWA – Health Canada is advising Canadians about the increased risk of opioid overdose and serious side effects when taking gabapentin (e.g., Neurontin) or pregabalin (e.g., Lyrica) with an opioid. Gabapentin is authorized to treat epilepsy and pregabalin is authorized to treat nerve pain. Both drugs belong to a class of drugs called gabapentinoids Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here. Gabapentin, initially developed for epilepsy, is now widely used for nerve pain and other off-label applications. Rising prescription rates have sparked discussions about whether it should be classified as a controlled substance due to concerns over misuse and dependency. National Regulation At the national level, gabapentin is not classified as a controlled substance under the Controlled Importing and exporting 6 (1) Except as authorized under the regulations, no person shall import into Canada or export from Canada a substance included in Schedule I, II, III, IV, V or VI. Marginal note: Possession for the purpose of exporting (2) Except as authorized under the regulations, no person shall possess a substance included in Schedule I, II, III, IV, V or VI for the purpose of Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab Act current to 2025-06-09 and last amended on 2025-04-14. Previous Versions Information on drug and health products authorized by Health Canada. Information on drug and health products authorized by Health Canada. The officer recognized the pill as gabapentin, which he had seen trafficked on the street with illegal drugs such as fentanyl and methamphetamine. Because the officer believed that gabapentin was a controlled drug under the CDSA, he immediately arrested the appellant for possession of a controlled substance. Regulations made under this Act Benzodiazepines and Other Targeted Substances Regulations (SOR/2000-217) Cannabis Regulations (SOR/2018-144) Controlled Drugs and Substances Act (Police Enforcement) Regulations (SOR/97-234) Narcotic Control Regulations (C.R.C., c. 1041) New Classes of Practitioners Regulations (SOR/2012-230) Precursor Control Regulations (SOR/2002-359) Precursors and Controlled Controlled Drugs and Substances Act S.C. 1996, c. 19 Assented to 1996-06-20 An Act respecting the control of certain drugs, their precursors and other substances and to amend certain other Acts and repeal the Narcotic Control Act in consequence thereof 1 NEURONTIN® (Gabapentin Capsules, 100 mg, 300 mg and 400 mg; Gabapentin Tablets, 600 mg and 800 mg), submission control 275525, Product Monograph, BGP Pharma ULC.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |