Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
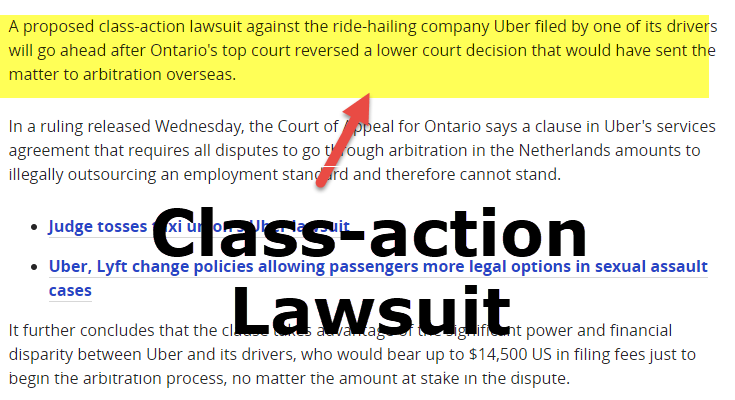
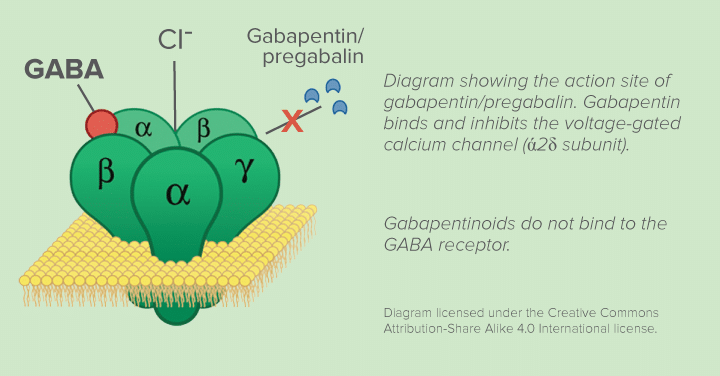
Neurontin class action settlement to compensate third-party payors who purchased, paid for, administered and/or reimbursed for gabapentin sold by Pfizer. Serving as co-lead counsel, Garwin Gerstein & Fisher LLP achieved a $190 million recovery for the Direct Purchaser Class. Direct purchasers claimed that Pfizer violated federal antitrust laws by illegally delaying the entry of generic versions of the prescription drug Neurontin. The active ingredient in Neurontin is gabapentin anhydrous. The lawsuit claimed that Pfizer delayed competition from A Diversified Practice The Schmidt Firm, PLLC has focused its practice on the representation of plaintiffs involved in both traditional personal injury and wrongful death litigation – involving medical malpractice, transportation accidents and nursing home abuse – as well as mass tort and toxic tort litigation – involving defective or dangerous pharmaceuticals, medical devices and toxic Los Angeles, CA: A $325 million preliminary settlement has been reached by Pfizer Inc and Warner-Lambert Co. LLC and plaintiffs who filed a consumer fraud class action lawsuit over the marketing UNITED STATES DISTRICT COURT FOR THE DISTRICT OF NEW JERSEY SUMMARY NOTICE OF PROPOSED CLASS ACTION SETTLEMENT, MOTION FOR ATTORNEYS’ FEES, AND HEARING REGARDING SETTLEMENT TO: ALL PERSONS OR ENTITIES WHO HAVE PURCHASED NEURONTIN DIRECTLY FROM PFIZER, INC. AND WARNER-LAMBERT AT ANY TIME DURING THE PERIOD OF DECEMBER 11, 2002, THROUGH AUGUST 31, 2008 AND WHO HAVE ALSO PURCHASED GENERIC If you or a family member has had any of the aforementioned Neurontin adverse effects, especially if it was administered for an unapproved purpose, you should contact an experienced class action attorney to talk about your case and learn more about your options for compensation for a Neurontin injury. The gabapentin class action lawsuit was filed in 2022 against three pharmaceutical companies – Teva, Pfizer, and Greenstone – accusing them of misrepresenting the drug’s risks and overstating its benefits. A $190 million settlement has been reached in New York in a consumer fraud class action lawsuit pending against Pfizer which alleges the pharma giant engaged in tactics to delay market entry of generic versions of its epilepsy drug Neurontin. The lawsuit was filed by purchasers of Neurontin in 2002, claiming Pfizer · The purpose of this notice is to alert you to a proposed settlement of a Class Action Lawsuit (the “Lawsuit”) brought by Direct Purchasers of Neurontin against Pfizer Inc. and Warner-Lambert Company LLC (collectively “Pfizer” or “Defendants”). The Lawsuit asserts that Pfizer violated antitrust laws relating to the sale of its prescription drug Neurontin. ACCLaws.com offers expert legal insights, case analyses, and updates on major lawsuits, helping you stay informed and legally empowered. An $85 million class action has been reached with Sun Pharmaceutical Industries and Taro Pharmaceuticals. The class action lawsuit claims that certain generic drug manufacturers broke antitrust laws, leading to artificially higher prices for common generic drugs such as amphetamine, amoxicillin, gabapentin, baclofen, and others. Neurontin Lawsuit | 2025 Latest Updates The prescription pain reliever Neurontin (generic gabapentin) has recently been linked to an increased risk for Stevens-Johnson syndrome (SJS), a severe skin disorder in which the top layer of the skin dies, followed by a painful rash that spreads and blisters. The manufacturers of the drug Neurontin reached a $325 million class action lawsuit settlement Wednesday over allegations they fraudulently marketed the prescription drug, which is used to treat seizures, restless leg syndrome, and pain caused by shingles. If approved, third-party payers of the drug will be eligible to claim a cash award from the Neurontin class action settlement, announced Pfizer, the world's largest drug maker, pleaded guilty on 13 May to numerous civil and criminal charges for illegally promoting the off-label use of gabapentin (Neurontin). It has agreed to pay a $240m (£136m; €200m) criminal fine and $152m to state and federal healthcare programmes. The fine is the second largest given in the industry. Meanwhile, off-label sales of gabapentin continue to Explore the potential for seeking compensation in gabapentin-related memory loss cases, including legal grounds, evidence, and filing deadlines. How To Join Gabapentin Lawsuit? In this insightful video, we delve into the process of joining a Gabapentin lawsuit. If you've been prescribed Gabapentin and believe you've suffered harm from its Hagens Berman brought suit against Pfizer and its subsidiary, Parke-Davis, accusing the companies of a fraudulent scheme to market and sell the drug Neurontin for a variety of "off-label" uses for which it is not approved or medically efficacious. Legal Urge offers daily legal insights, news, and analysis to simplify complex laws. Explore expert content that informs, empowers, and sparks legal curiosity. Pfizer Inc. has agreed to a $190 million class action settlement that, if approved, would resolve claims that it delayed generic versions of its epilepsy drug Neurontin and promoted it for unapproved uses. The class action lawsuit was initially filed more than a decade ago by direct purchasers of Neurontin. The plaintiffs alleged that Pfizer violated the Sherman Act by maintaining a its The settlement centered on Pfizer’s fraudulent marketing of Neurontin for off-label uses not approved by the Food and Drug Administration (FDA). Another significant case occurred in 2010 when the company agreed to pay $325 million to settle a class-action lawsuit brought by third-party payers.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |