Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 | |
 |  |
 |  |
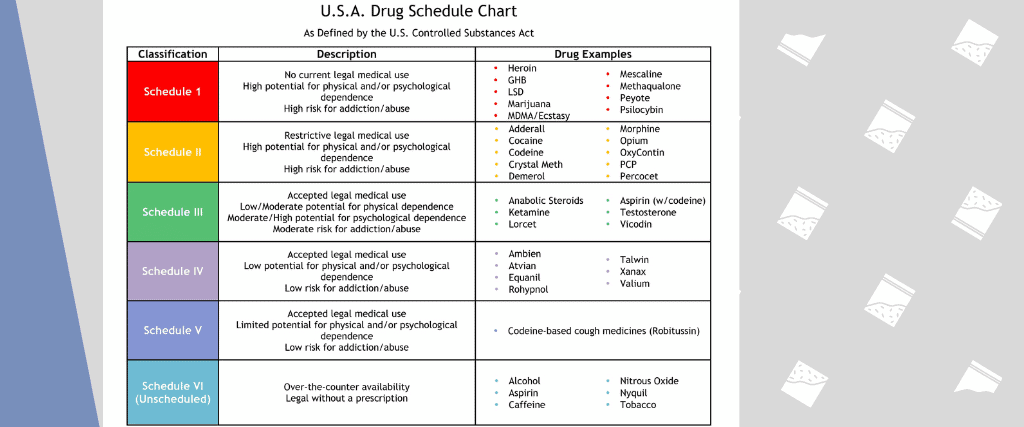
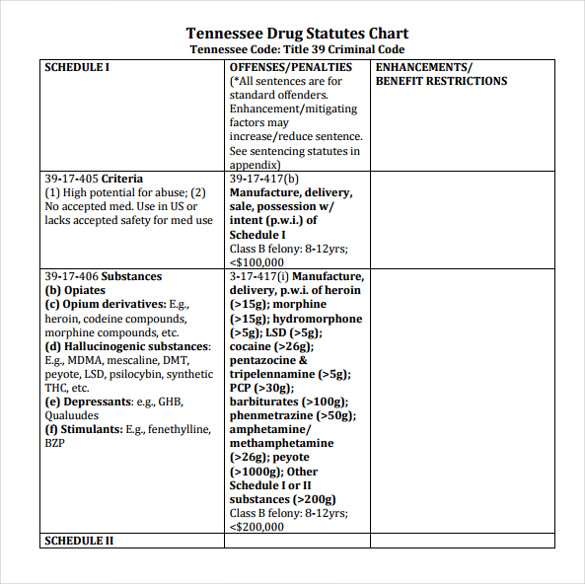
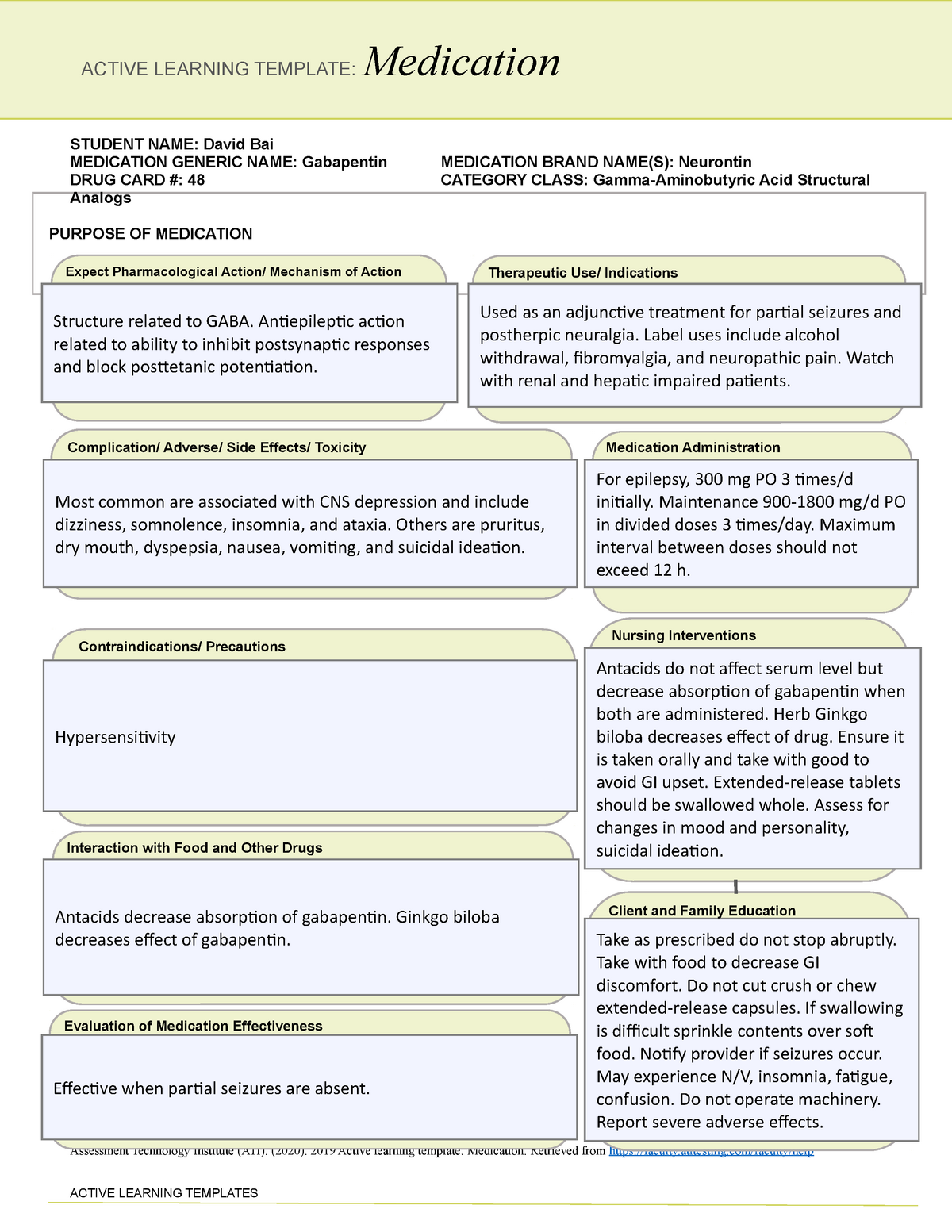
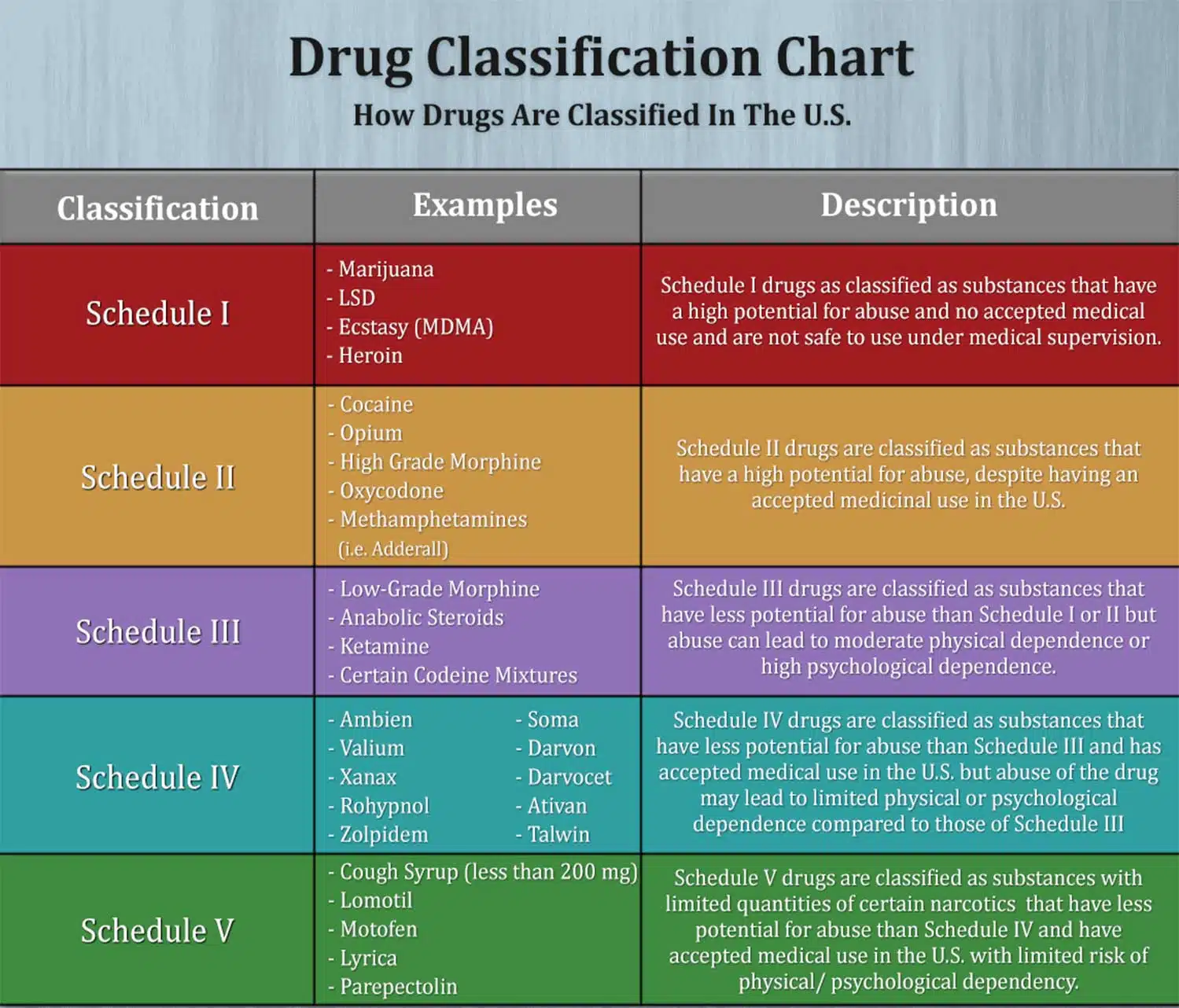
Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create We would like to show you a description here but the site won’t allow us. *These are drug products which: (1) may be dispensed only upon a prescription issued by a practitioner and, (2) contain controlled substances but have been specifically excepted from the controlled substances schedules. (Title 21, CFR 1308.31.) Accordingly, these drugs are legally classified as dangerous drugs in Ohio. Rx-Prescription Drugs. Gabapentin is a prescription medication that falls into a class of drugs known as anticonvulsants, sometimes called anti-epileptic drugs. It works by reducing specific nerve signals sent to the brain, helping you cope with chronic pain and improve alertness. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. We would like to show you a description here but the site won’t allow us. See Epilepsy. MHRA/CHM advice: Gabapentin (Neurontin ®) and risk of abuse and dependence: new scheduling requirements from 1 April (April 2019) Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Briefing Note From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. The Controlled Substances Act (CSA) categorizes drugs into schedules based on their medical use, safety, and potential for abuse. Schedule I drugs are considered the most dangerous with no accepted medical use, while Schedule V drugs are deemed to have a lower potential for abuse. Gabapentin’s classification varies by state. Pharmacies licensed and located in Minnesota must report to the MN PMP all schedule II-V controlled substance prescriptions, along with prescriptions for butalbital and gabapentin, when dispensed. II: N Adzenys ER/XR-ODT, Dyanavel XR, Evekeo, Evekeo ODT Anileridine 9020 II Y Leritine Benzhydrocodone combination products 9193 II Y Apadaz Bezitramide 9800 II Y Burgodin Carfen Gabapentin is not a narcotic or federally controlled substance, but it is classified as a Schedule V drug in certain states due to its potential for abuse and diversion. Learn which states control gabapentin, why it is regulated, and how it can interact with opioids and other drugs. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Why does gabapentin’s drug class vary from state to state? Although gabapentin isn’t controlled federally, some states have listed it as a controlled substance and therefore regulate its use. All applicable provisions of recently passed Utah House Bill 260 CONTROLLED SUBSTANCES AMENDMENTS that a ected UCA 58-37-4 Schedules of controlled substances and other licensure board regulations will apply to Gabapentin. Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk of overdose death when used in combination with them. The US has seen substantial increases in The Schedule V designation reflects a lower potential for abuse compared to higher-scheduled drugs but still warrants oversight. Gabapentin is monitored through the Tennessee Controlled Substance Monitoring Database (CSMD), which requires all dispensations to be reported.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 | |
 |  |
 |  |