Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 | |
 |  |
A federal appeals court has upheld a $142 million jury verdict in a case involving Pfizer’s epilepsy drug Neurontin, and opened the door for potentially even more lawsuits. On April 3, the U.S Seeing how gabapentin works, it is not terribly surprising that memory loss is a possible side effect. It is though that part of the mechanism of action of gabapentin contributes to some mild central nervous system depression. Not only can this cause slight memory loss, but it can also cause movement problems, slurred speech etc A federal jury in Boston has found that Pfizer violated U.S. racketeering laws by illegally promoting off-label uses of its epilepsy drug Neurontin, and the drug maker may be forced to pay more Memory loss related to gabapentin use may range from mild forgetfulness to noticeable difficulties with recall or focus. For some, these changes may resolve as the body adjusts to the medication, while for others, they might persist, requiring medical attention. Explore the potential for seeking compensation in gabapentin-related memory loss cases, including legal grounds, evidence, and filing deadlines. Explore gabapentin's effects on mental function, memory, and cognition. Learn about managing side effects and balancing therapeutic benefits with potential risks. Pfizer, the world's largest drug maker, pleaded guilty on 13 May to numerous civil and criminal charges for illegally promoting the off-label use of gabapentin (Neurontin). It has agreed to pay a $240m (£136m; €200m) criminal fine and $152m to state and federal healthcare programmes. The fine is the second largest given in the industry. Meanwhile, off-label sales of gabapentin continue to Cox proportional hazards regression models were used to investigate the associations between exposure to gabapentin or pregabalin and the quartiles of cDDDs of gabapentin or pregabalin exposure and the risk of dementia, adjusting other potential confounders and estimating the hazard ratios (HR) and 95% confidence intervals (CIs). Discover whether you can reverse memory loss from gabapentin and restore cognitive clarity. Explore the potential for reclaiming your memory. Gabapentin Memory Loss Lawsuit One of the main allegations in the gabapentin class action is that the drug manufacturers failed to warn about the risk of memory loss associated with the anticonvulsant medication. Neurontin Lawsuits Neurontin (generic name: gabapentin) was FDA-approved in 1993 for use as an anticonvulsant for people suffering from partial seizures associated with epilepsy. A $325 million Neurontin class action lawsuit settlement is reached with third-party payors of the drug, just months after a $190 million Neurontin settlement is reached with direct purchasers. Public Citizen has petitioned the FDA to classified gabapentin, the active ingredient in Neurontin and other, similar epilepsy drugs, as a controlled substance due to increasing drug abuse problems. Pfizer Inc has agreed to pay $325 million to resolve claims it defrauded insurers and other healthcare benefit providers by marketing Neurontin for unapproved uses, its second settlement over the A Gabapentin lawsuit was filed in 2022, alleging that the manufacturers of Gabapentin failed to warn patients about the potential side effects of the medication, particularly memory loss. However, the overall benefits of Gabapentin in treating various conditions cannot be overlooked. It gives me extreme Vertigo. I am prescribed Gabapentin by the VA in Manchester NH, since taking the medication I have memory loss, depression and weight gain. Plaintiffs allege that Pfizer violated federal antitrust laws by illegally delaying the entry of generic versions of the prescription drug Neurontin. The active ingredient in Neurontin is gabapentin anhydrous. Plaintiffs allege that Pfizer delayed competition from less expensive generic versions of Neurontin by executing a multifaceted scheme involving, among other things, improperly listing Federal prosecutors on Thursday announced that Pfizer has agreed to plead guilty to criminal wrongdoing and pay a settlement of $430 million in a whistleblower lawsuit alleging that the Parke-Davis unit of Pfizer subsidiary Warner-Lambert illegally encouraged physicians to prescribe the epilepsy drug Neurontin to treat conditions other than those approved by FDA, USA Today reports. Warner-Lambert agreed on May 13 to plead guilty and pay more than $430 million to resolve criminal charges and civil-health-care liabilities in connection with its Parke-Davis division's “illegal and fraudulent promotion of unapproved uses” of Neurontin (gabapentin). In a pointed, detailed press release, US Department of Justice officials document the company's “widespread, coordinated
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
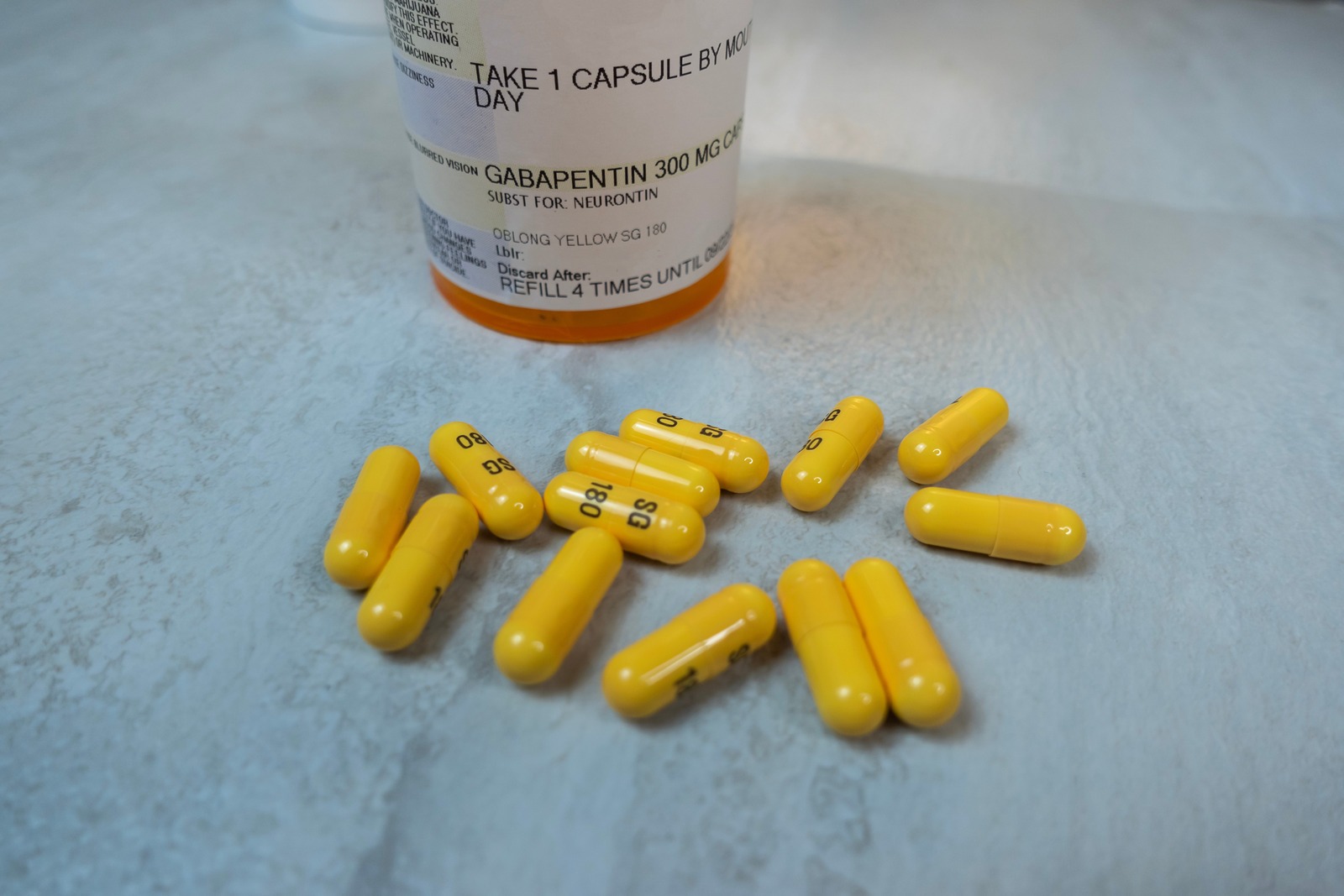 |  |
 | |
 |  |