Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
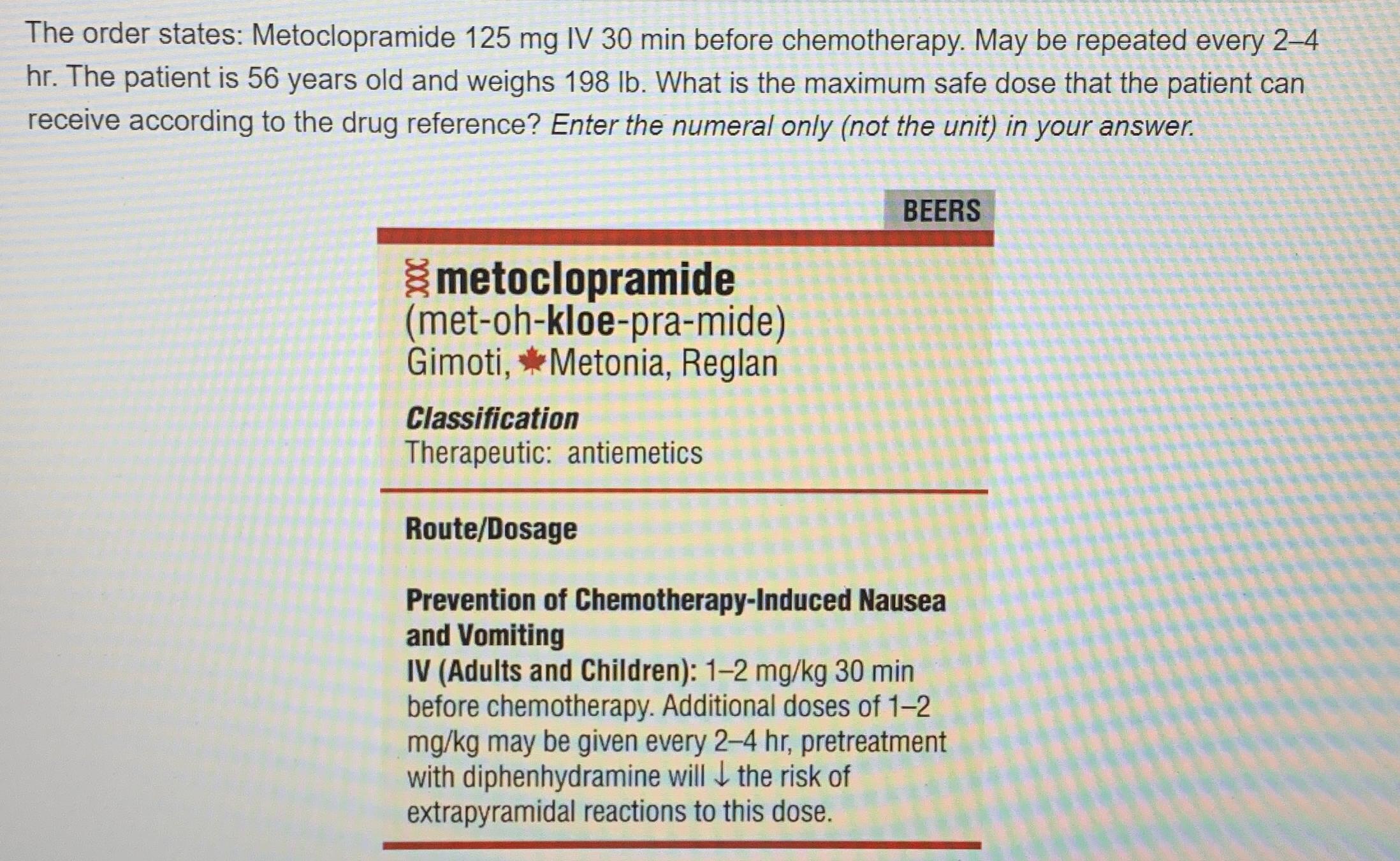
The MHRA and manufacturers advise that when prescribing gabapentin in patients who require concomitant treatment with opioid medicines, patients should be carefully observed for signs of CNS depression, such as somnolence, sedation, and respiratory depression, and the dose of either gabapentin or the opioid should be reduced appropriately.6,7 View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. Safety leaflet to help you understand the latest information about the effects of pregabalin during pregnancy. Evaluate patients carefully for a history of drug abuse before prescribing pregabalin and gabapentin and observe patients for development of signs of abuse and dependence. The MHRA concluded that the use of Pregabalin for all licensed indications during the first trimester of pregnancy may result in a slightly increased risk of major congenital malformations in the unborn child, compared to contemporaries. Crescent Pharma Limited has informed the MHRA of an error with the European Article Number (EAN) barcode on the cartons of a batch of simvastatin 10 mg Tablets distributed by Alliance Healthcare In this monthly update, we highlight selected Medical Device Alerts that have been issued recently by MHRA. Please note, this is not an exhaustive list of medical device alerts. From April 2019, due to the risk of dependence and misuse, gabapentinoids were re-classified as Class C controlled substances and Schedule 3 controlled drugs under the Misuse of Drug Regulations 2001. There have been other MHRA alerts for gabapentinoids, including: In a recent Drug Safety Update, the Medicines and Healthcare products Regulatory Agency (MHRA) warned about a rare risk of severe respiratory depression with gabapentin, with or without concomitant use of opioids. 1 In England, 6.5 million prescriptions for gabapentin were dispensed in 2016. 2 CAS-ViewAlertView Alert Gabapentin has been associated with a rare risk of severe respiratory depression even without concomitant opioid medicines. Report a side effect with a medicine or medical device. Loading results The product information for gabapentin, approved by the Medicines and Healthcare products Regulatory Agency (MHRA), provides information to support the prescribing of the medication including recommendations for dose, duration of use, as well as gradual reduction when stopping. The MHRA 1 highlights the significant safety concerns and risks of respiratory depression associated with gabapentin use. Morphine can increase the bioavailability of gabapentin. Caution is needed when these drugs are co-prescribed and the doses of both drugs may need to be modified. Pregabalin has been associated with infrequent reports of severe respiratory depression, including some cases without the presence of concomitant opioid medicines. Patients with compromised Based on the review of the data on quality, safety and efficacy, the Medicines and Healthcare products Regulatory Agency (MHRA) considered that the applications for Gabapentin Strides 100, 300 and 400 mg capsules, hard (PL 13606/0232-34) could be approved. The Medicines and Healthcare First, we inform clinicians of the findings of a comprehensive safety review of products Regulatory Agency antiepileptic drugs in pregnancy. We ask clinicians to use this information when (MHRA) is the government agency responsible for discussing treatment options with women with epilepsy at initiation and at ensuring that medicines and routine recommended annual Gabapentin and Pregabalin prescribing for neuropathic pain. Prescribing in patients aged 65 years or over. Gabapentin and Pregabalin prescribing for neuropathic pain. Prescribing in patients aged 65 years or over. A safety review has been conducted by the MHRA following a Yellow Card report concerning a patient who was taking bromocriptine. The review concluded that blood pressure monitoring of patients Medical Device Alerts issued in September 2017 In this monthly update, we highlight selected Medical Device Alerts that have been issued recently by MHRA. Please note, this is not an exhaustive list of medical device alerts. For all Medical Device Alerts from MHRA, see Alerts and recalls for drugs and medical devices.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |