Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
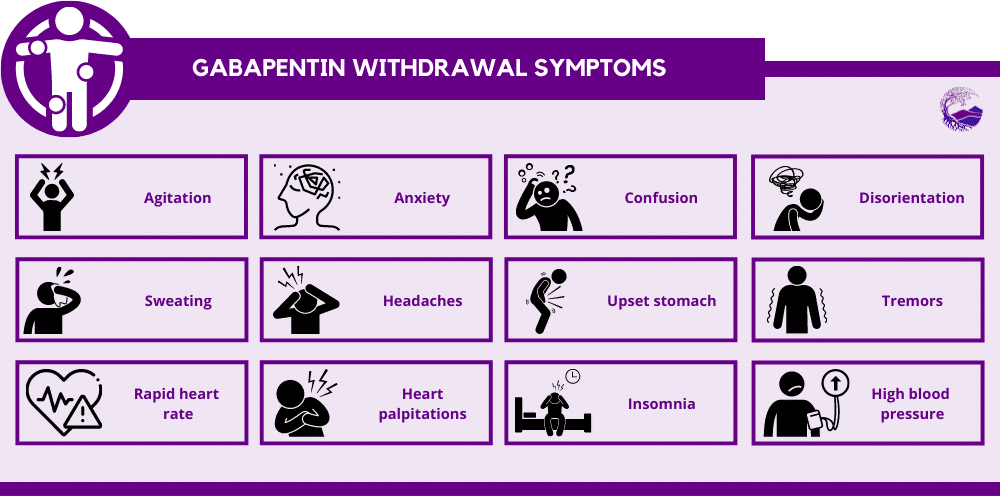
This means gabapentin has a lower risk of abuse compared to Oxycontin (oxycodone), which is a schedule II opioid medication. At this time, seven states label gabapentin as a controlled substance. As healthcare professionals, Pharmacists play a very critical role in helping Arizona solve the prescription drug misuse and abuse problem in our state. These guidelines are intended to help Dispensers reduce the inappropriate use of controlled substances while preserving the vital role of the Pharmacy to treat patients with medical conditions. These guidelines were developed at the Arizona We would like to show you a description here but the site won’t allow us. Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. Arizona Prescription Monitoring Program The Arizona Prescription Monitoring Program (PMP), housed in the Arizona State Board of Pharmacy, collects data on all controlled substance prescriptions in Arizona (schedules II-V). This information assists healthcare providers in making better-informed care decisions when treating patients. The number of states placing gabapentin on the controlled substance list or in their required monitoring program is growing and three more states are debating whether to add gabapentin as a controlled substance or to their mandated reporting programs (DE, NY, and WI). Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. 3. "Board" means the Arizona state board of pharmacy. 4. "Cannabis" means the following substances under whatever names they may be designated: (a) The resin extracted from any part of a plant of the genus cannabis, and every compound, manufacture, salt, derivative, mixture or preparation of such plant, its seeds or its resin. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab There have been increasing reports of gabapentin misuse, either alone or with opioids to enhance their euphoric (“high”) effects. At the federal level, gabapentin is not a controlled substance. However, some states have implemented their laws to reclassify gabapentin as a Schedule V controlled substance. Purpose Requires the Arizona State Board of Pharmacy (Board) to adopt the Schedule I, II, III, IV and V controlled substance designations as listed in federal law. Here's who gabapentin was originally approved for, what it's used for today and why it's becoming a drug of increasing concern for abuse and misuse. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. 1. A schedule II or schedule III controlled substance as defined in the controlled substances act (P.L. 91-513; 84 Stat. 1242; 21 United States Code section 802) without board approval and United States drug enforcement administration registration. If the physician assistant has less than eight thousand clinical practice hours, the supervision agreement shall specify the physician assistant's Background Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear. Objective To determine the effect of state-imposed Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. The abuse potential of gabapentin is well documented; with gabapentin having been noted as an agent highly sought after for use in potentiating opioids. When combined with opioids, the risk of respiratory depression and opioid-related mortality Gabapentin is a non-opioid pain medication for treating neuropathy and seizures caused by epilepsy. Although rates of gabapentin misuse are rising, the drug is not federally regulated as a controlled substance. Several states, however, have enacted laws in recent years to list gabapentin as a controlled substance. Arizona Controlled Substances Prescription Monitoring Program Electronic Prescribing of Controlled Substances (EPCS) Pharmacy Requirements Beginning January 1, 2020, a schedule II controlled substance that is an opioid may be dispensed only with an electronic prescription order as prescribed by federal law or regulation.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |