Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 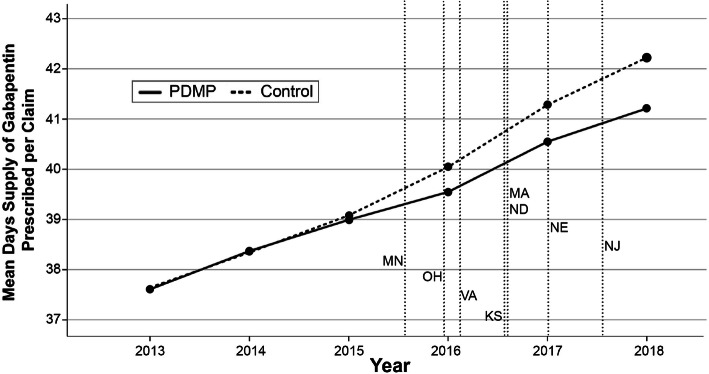 |
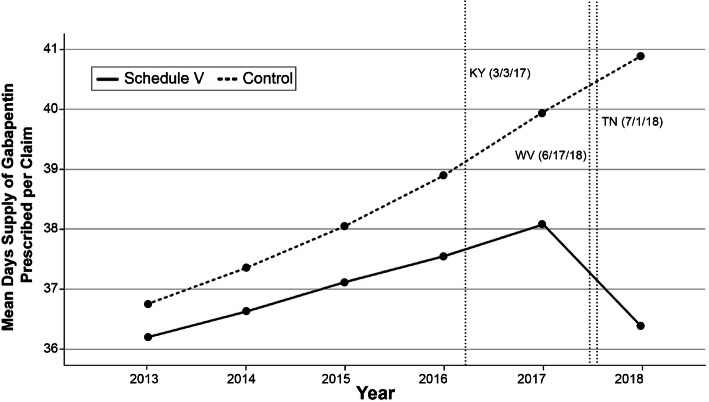
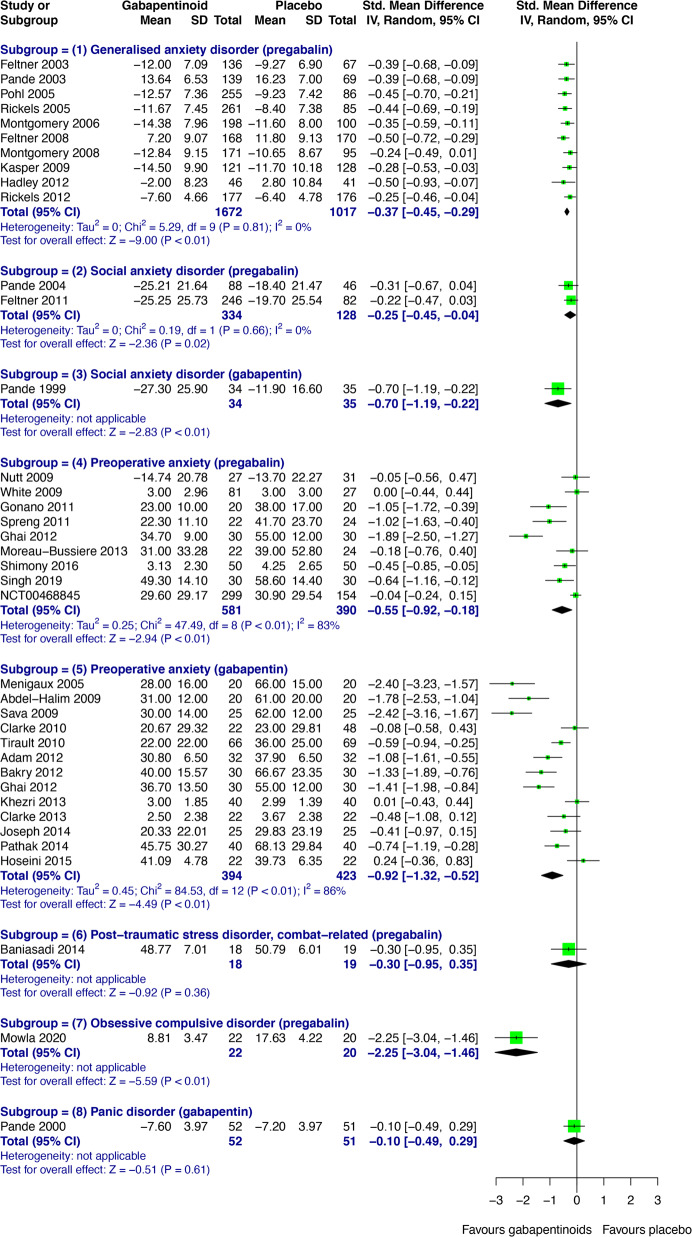
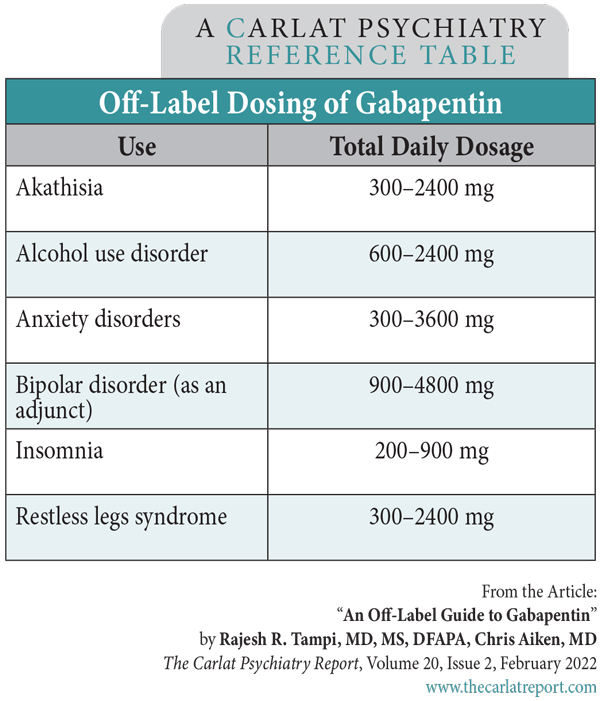
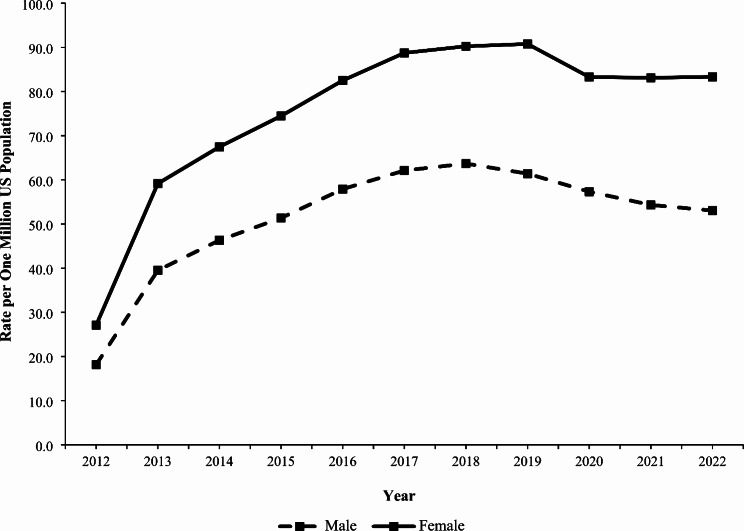
Gabapentin isn't a narcotic, but it is a controlled substance in some states. Here's what you should know before using it. Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial Each state may regulate gabapentin slightly differently, setting its own time, quantity, or dosage limits to minimize the risk of drug misuse and abuse. For example, Rhode Island law only allows up to a 90-day supply for prescriptions for Schedule V controlled substances. Gabapentin is a Schedule V drug in states where it’s classified as a controlled substance. Despite its increasing use, especially for off-label purposes, gabapentin typically does not have the same potential for misuse or dependence as some other drugs, such as opioids or benzodiazepines. While gabapentin is not a Federal Schedule V Controlled Substance, these recent actions taken by individual states should alert pharmacists about poten- tial risks related to the drug and high- light the possible need for additional patient counseling in some cases. If gabapentin is, or becomes, a controlled substance in your state, it does not necessarily mean it will be more difficult to obtain. Rather, it is a safety measure to assure we are using medications appropriately. Talk with your doctor or pharmacist if you have questions or aren’t sure if gabapentin is working for you. RX DRUG SCHEDULING & MONITORING Prescription Drug Monitoring Programs (PDMPs) are electronic databases that collect information on the dispensing and prescribing of drugs within jurisdictions. PDMPs aim to assist patients in their quality of care by allowing prescribers and dispensers access to the patient’s controlled substance prescription medication history. This access to individual Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Gabapentin is a medication for nerve pain and seizures. It’s not a controlled substance by the federal government, but some states classify it as a schedule V drug. Learn why gabapentin is risky and how to avoid misuse. Gabapentin – or Neurontin – is a medication commonly used to treat nerve pain and seizures. However, the drug can have potentially harmful effects when combined with other opioids. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. Important notice regarding changes to the law affecting prescribing practitioners: Effective July 1st, 2019: pursuant to House Enrolled Acts 1246 and 1542, the Indiana scheduled prescription electronic collection and tracking (INSPECT) program will begin monitoring and tracking the prescribing and dispensation of Gabapentin. Please note, this change to statute solely provides for the Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation Classifying gabapentin as a Schedule V drug results in substantial reduction in total days prescribed whereas PDMP regulation results in modest reduction. PDF RCW 69.50.212 Schedule V. Unless specifically excepted by state or federal law or regulation or more specifically included in another schedule, the following controlled substances are listed in Schedule V: Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Schedule V Controlled Substance in Tennessee July 1, 2018 Effective July 1, 2018, all gabapentin products became Schedule V controlled substances in the state of Tennessee. Gabapentin is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is not a narcotic or federally controlled substance, but it is classified as a Schedule V drug in certain states due to its potential for abuse and diversion. Learn which states control gabapentin, why it is regulated, and how it can interact with other drugs.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |