Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
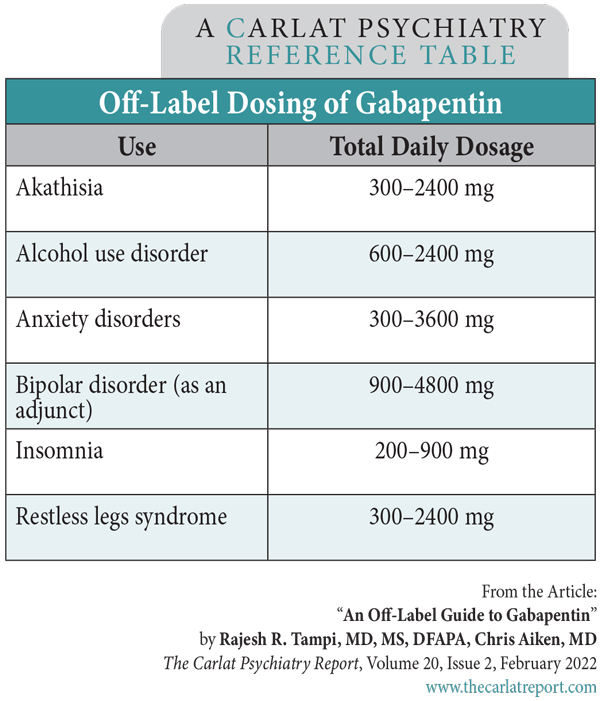
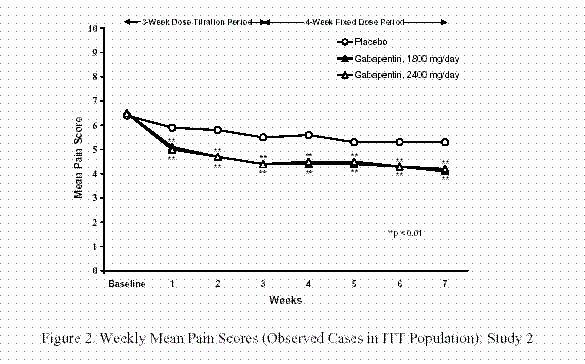
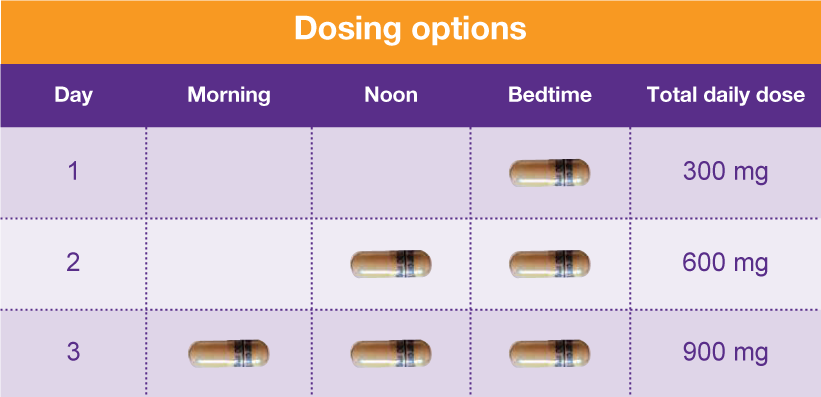
II: N Adzenys ER/XR-ODT, Dyanavel XR, Evekeo, Evekeo ODT Anileridine 9020 II Y Leritine Benzhydrocodone combination products 9193 II Y Apadaz Bezitramide 9800 II Y Burgodin Carfen Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. Accordingly, this study aims to explore the experiences and perceptions of Kentucky community pharmacists regarding (1) gabapentin misuse and diversion, (2) current pharmacy policies affecting gabapentin dispensing, and (3) their support or opposition to regulatory changes that reclassify gabapentin as a schedule V CS in Kentucky. Kentucky - Edition 52 Important Notice: Gabapentin Becomes A Schedule 5 Controlled Substance In Kentucky 2017-08-12 00:51:38 Office of Inspector General (OIG), Drug Enforcement and Professional Practices Branch (DEPP) Effective July 1, 2017, all gabapentin products will be Schedule 5 controlled substances in Kentucky. All applicable provisions of KRS Chapter 218A, 902 KAR Chapter 55 and other Owing to increasing concern over the potential for gabapentin misuse, gabapentin was reclassified as a schedule V controlled substance in Kentucky (July 2017). The Cabinet for Health and Family Services recognizes that nalbuphine and gabapentin have significant abuse potential, and inclusion on Kentucky's controlled substances schedules will help reduce the risk to public health. Section 1. Schedule I Controlled Substances. (1) Methods This study used Kentucky All Schedule Prescription Electronic Reporting data (2018). Gabapentin use was defined as having at least 1 dispensed gabapentin prescription, and high-dose gabapentin use was defined as an average daily dose of more than 3600 mg at the patient level. Gabapentin now Controlled Substance As of July 1, 2017, gabapentin will be classified as a Schedule V, controlled substance (in KY). APRNs must now meet prescribing requirements for controlled substances in order to prescribe gabapentin. APRNs will no longer be able to prescribe gabapentin unless they have a DEA license and a CAPA-CS. The first is to determine whether gabapentin-involved overdose deaths changed in Kentucky after it became a Schedule V drug in July 2017. She will accomplish this by comparing data from 12 months before and 12 months after the drug was scheduled. Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here. Kentucky will become the first state to change gabapentin to a Schedule V controlled substance, effective July 1, 2017. Current FDA-approved indications for gabapentin are post-herpetic neuralgia and seizures. Important Notice: Gabapentin Becomes a Schedule 5 Controlled Substance in Kentucky Effective July 1, 2017, all gabapentin products will be Schedule 5 controlled substances in Kentucky. All applicable provisions of KRS Chapter 218A, 902 KAR Chapter 55 and other licensure board regulations will apply to gabapentin. We would like to show you a description here but the site won’t allow us. We compared two interventions including (1) class V scheduling of gabapentin and (2) gabapentin inclusion in the state PDMP using a control group of similar states. The Schedule V change for gabapentin was implemented in three states including Kentucky, Tennessee, and West Virginia (Table 1). Owing to increasing concern over the potential for gabapentin misuse, gabapentin was reclassified as a schedule V controlled substance in Kentucky (July 2017). As of November 2020, seven states — Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia — had classified gabapentin as a schedule V drug, while another 12 states Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. The federal regulation designates these substances as a Schedule IV controlled substance. The Cabinet for Health and Family Services recognizes that pentazocine and derivatives of barbituric acid or its salts have significant abuse potential, and inclusion on Kentucky’s Schedule III list will help reduce the risk to public health. In Kentucky, no mid-level practitioners are authorized to directly dispense controlled substances. Practitioners who directly dispense gabapentin FROM their stock TO a patient, including both administering and dispensing, shall transmit the required dispensing data to the KASPER system in accordance with KRS 218A.202 and 902 KAR 55:110. Gabapentin is widely prescribed in Kentucky, with higher rates of use observed in females, those older than 55 years and individuals living in the Appalachian region. Concurrent use of gabapentin and OAs is common, especially in those receiving high-dose gabapentin. Future studies are needed to asse
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |