Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
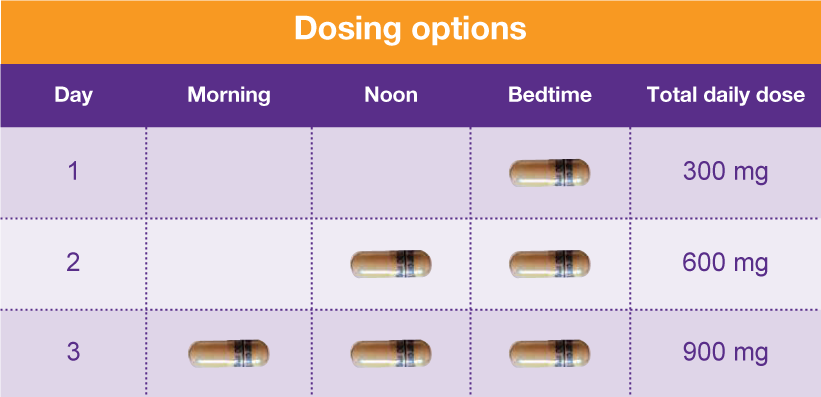
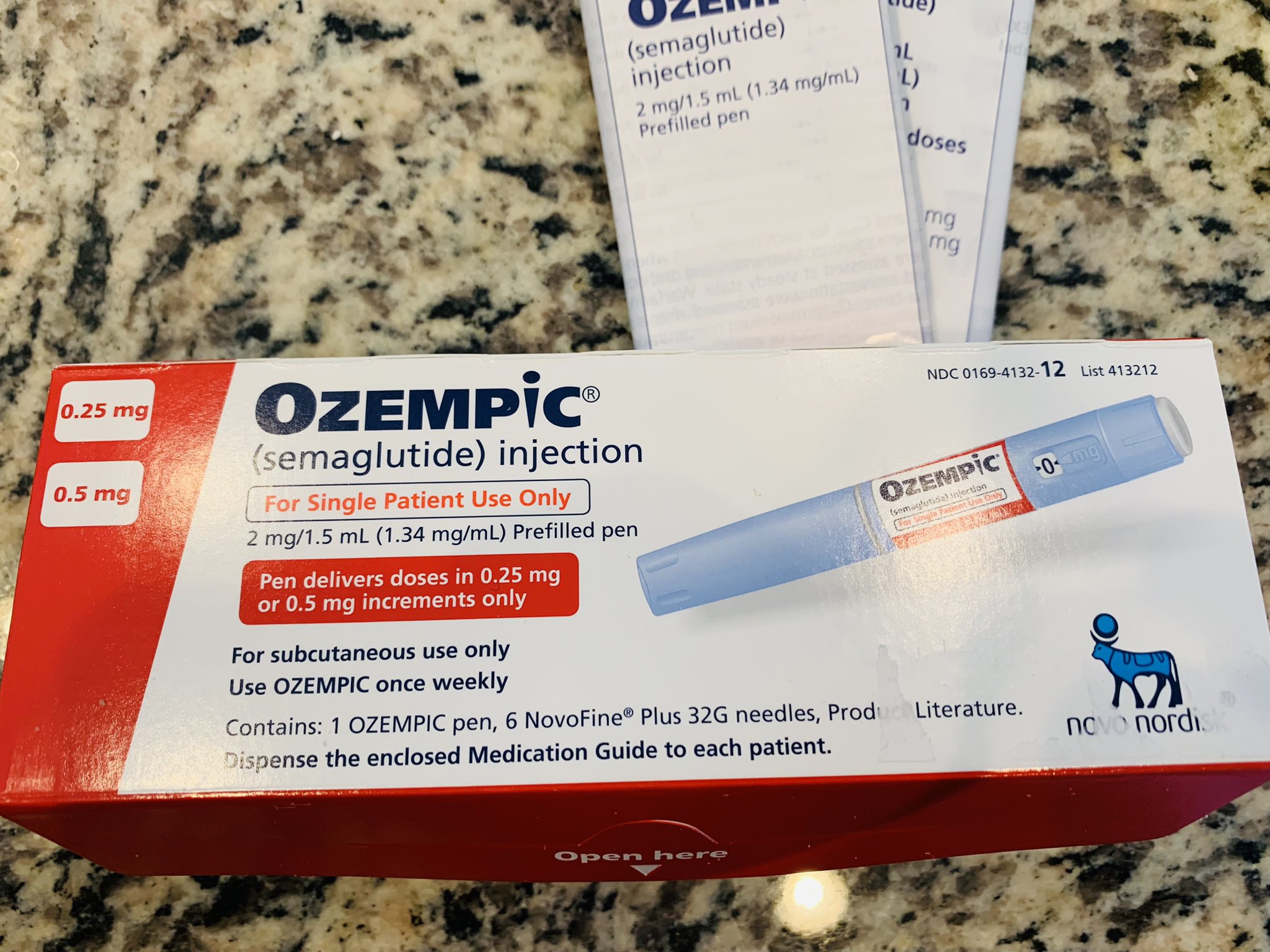
The Texas Prescription Monitoring Program (PMP) collects and monitors prescription data for all Schedule II, III, IV, and V Controlled Substances (CS) dispensed by a pharmacy in Texas or to a Texas resident from a pharmacy located in another state. The PMP also provides a database for monitoring patient prescription history for practitioners and the ordering of Texas Schedule II Official Access the current Texas controlled substance schedule. Stay up-to-date with the most recent changes to the schedule and explore previous schedules. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. Neurontin (gabapentin) Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria We would like to show you a description here but the site won’t allow us. SCHEDULES OF CONTROLLED SUBSTANCES PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES, ESTABLISHED JANUARY 1, 2001, SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was signed by Jennifer A. Shuford, M.D., M.P.H., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedati Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. The Texas Prescription Monitoring Program (PMP) is an electronic database that collects and monitors outpatient prescription data for Schedule II, III, IV, and V controlled substances dispensed by pharmacies in Texas, or to a Texas resident by a pharmacy in another state. “We’ve seen a significant increase in gabapentin prescribing over the last 10 to 15 years, but is it actually helping patients? The data suggest that it prob- ably isn’t, and that’s a major concern for me,” said Jordan Covvey, PharmD, PhD, an associate professor in the School of Pharmacy at Duquesne University in Pittsburgh. While gabapentin is not a Federal Schedule V Controlled Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). In 1981, the Texas Legislature passed a law which required doctors to write all prescriptions for Schedule II drugs on a special three‑part or triplicate form. Effective September 1, 1999, the triplicate prescription form was replaced by an official prescription form. Effective June 1, 2019, only an official prescription form is valid. Texas Health and Safety Code HEALTH & SAFETY TX HEALTH & S Section 481.104. Read the code on FindLaw Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Key takeaways: Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule Schedules of Controlled Substances Controlled Substances Are Categorized by Addiction and Abuse Potential Federal and Texas law organizes controlled substances into five schedules or categories based on their potential for addiction and abuse. Drugs that are a greater threat are classified in Schedule I while the least dangerous drugs are put into Schedule V. Neurontin® and generic gabapentin immediate-release dosing is FDA-approved for use as adjunctive therapy for partial seizures with or without generalization in pediatric epileptic patients 12 years of age and older, as well as adjunctive therapy for partial seizures in pediatric patients 3 years to 11 years of age.1-3, Gabapentin extended
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |