Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
:max_bytes(150000):strip_icc()/VWH-GR_Fran_Drescher_03_0914_rgb1500x1000-d07e2408f2ba44b08fbe8959d0b0f87c.jpg) |  |
 |  |
 |  |
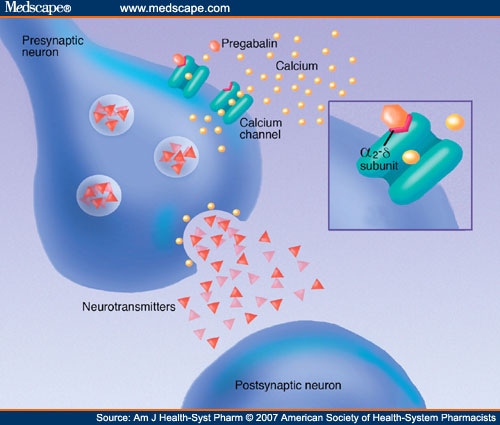
Briefing Note From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Gabapentin is classified as a controlled substance in several states, including Alabama, Georgia, Kentucky, Tennessee, and Texas. These states have placed it under Schedule V, indicating a lower potential for abuse compared to higher schedules. GABAPENTIN (Trade Name: Neurontin®) Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. Unless listed in another schedule, a material, compound, mixture or preparation that contains any quantity of the following substances having a depressant effect on the central nervous system, including the substance's salts, isomers, and salts of isomers if the existence of the salts, isomers, and salts of isomers is possible within the Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk of overdose death when used in combination with them. The US has seen substantial increases in The schedule depends on the drug’s potential for abuse or dependency. Schedule V drugs have a lower potential for abuse than all other controlled substances. This means gabapentin has a lower risk of abuse compared to Oxycontin (oxycodone), which is a schedule II opioid medication. Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation We would like to show you a description here but the site won’t allow us. Key takeaways: Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create A structured gabapentin taper chart helps ease withdrawal and minimize risks, but knowing what works—and what doesn’t—matters just as much. Learn more. Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. II: N Adzenys ER/XR-ODT, Dyanavel XR, Evekeo, Evekeo ODT Anileridine 9020 II Y Leritine Benzhydrocodone combination products 9193 II Y Apadaz Bezitramide 9800 II Y Burgodin Carfen RX DRUG SCHEDULING & MONITORING Prescription Drug Monitoring Programs (PDMPs) are electronic databases that collect information on the dispensing and prescribing of drugs within jurisdictions. PDMPs aim to assist patients in their quality of care by allowing prescribers and dispensers access to the patient’s controlled substance prescription medication history. This access to individual ver view ective May 1, 2024, all Gabapentin products will be Schedule V controlled substances in Utah. View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Gabapentin, initially developed for epilepsy, is now widely used for nerve pain and other off-label applications. Rising prescription rates have sparked discussions about whether it should be classified as a controlled substance due to concerns over misuse and dependency.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
:max_bytes(150000):strip_icc()/VWH-GR_Fran_Drescher_03_0914_rgb1500x1000-d07e2408f2ba44b08fbe8959d0b0f87c.jpg) |  |
 |  |
 |  |