Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
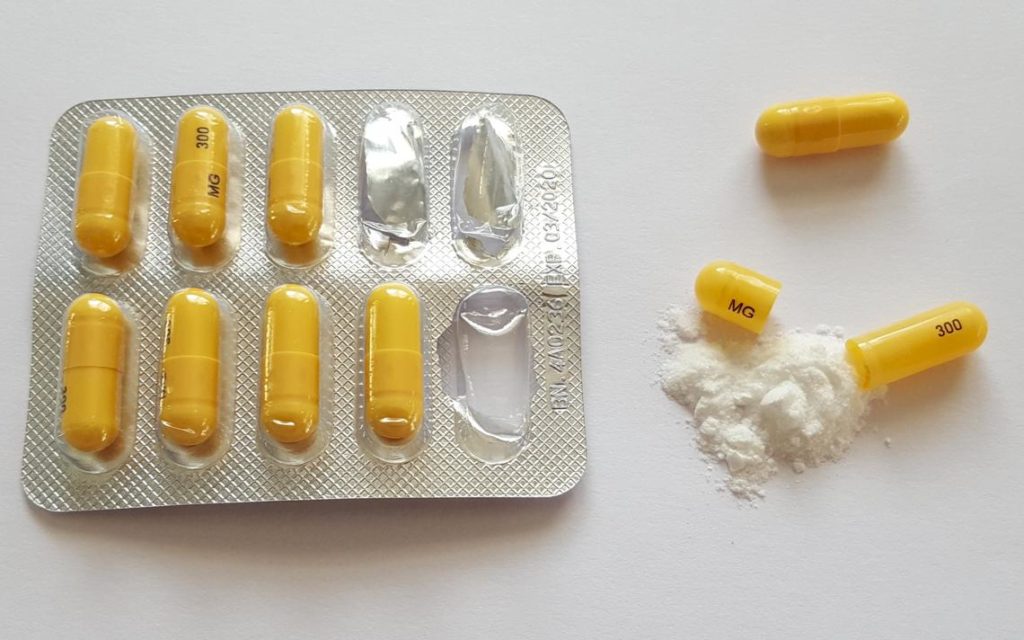
On July 2, 2018, the Rhode Island Department of Health (RIDOH) made regulatory changes to the Rules and Regulations for Pain Management, Opioid Use, and the Registration of Distributors of Controlled Substances in Rhode Island [216-RICR-20-20-4]. These regulations require prescribers to: 1. (b) The schedules I, II, III, IV, and V shall, unless and until amended pursuant to this chapter, consist of: those enumerated in this article and include substances to be controlled by rule and/or regulation of the director of health as published, except all substances in schedules II, III, IV, and V will require a prescription to be dispensed by an apothecary in the state of Rhode Island Rhode Island enforces strict controlled substance laws regulating possession, distribution, and use of drugs. These laws aim to prevent abuse while allowing legitimate medical use under regulated conditions. With mounting evidence of misuse and abuse of gabapentin use, certain states have implemented regulations or policies to limit or monitor the use of the drug, especially given its potential to enhance the effects of opioids. Some experts and nonprofit groups have called for national reclassification of gabapentin as a controlled substance. Gabapentin is not classified as a controlled substance on the federal level. However, there are growing concerns about the potential risks. Here’s what to know. 2021 Rhode Island General Laws Title 21 - Food and Drugs Chapter 21-28 - Uniform Controlled Substances Act The Rhode Island PDMP collects data on all controlled substances (schedules II-V) and opioid agonist prescriptions that are dispensed by retail pharmacies with a controlled substance registration in Rhode Island or to Rhode Island residents. Complete data exists from April 1, 2016 to present. Incomplete data exist from February 2010 to March 2016. Background and Purpose In Rhode Island, after seeing a decrease by 8.3% from 2016 to 2019, accidental drug overdose deaths increased by 25%, from 308 in 2019 to 384 in 2020. Illicit drugs and controlled substance prescriptions have both contributed to drug overdose deaths in our state. Currently, all dispensing of controlled substances in schedules II-V must be reported, pursuant to Section 21a-254(j) of the Connecticut General Statutes, into the CPMRS. Under Section 21a-254(j)(2) the Commissioner of Consumer Protection has the authority to add any products or substances to the CPMRS. Adding Gabapentin to the CPMRS will provide an additional data point to assist prescribers The Rhode Island Prescription Drug Monitoring Program (PDMP) collects data for controlled substance prescriptions (Schedules II - V, or opioid antagonists) into a centralized database. Abstract The abuse potential of gabapentin is well documented; with gabapentin having been noted as an agent highly sought after for use in potentiating opioids. When combined with opioids, the risk of respiratory depression and opioid-related mortality increases significantly. In the US, gabapentin was approved by the Food and Drug Administration as a non-controlled substance. To date, and in Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. January 2025 According to Rhode Island General Law, the Director of the Rhode Island Department of Health (RIDOH) recognizes the current version of Title 21 of the Code of Federal Regulations (CFR) as the most updated list of controlled substances in Rhode Island. Controlled Substances The federal government, through the Controlled Substances Act makes classified drugs, substances, and certain chemicals used to make drugs, into five distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. Title 21 Food and Drugs Chapter 28 Uniform Controlled Substances Act Article IV Offenses and Penalties (a) This chapter may be cited as the “Rhode Island Controlled Substances Act”, and shall be so interpreted and construed as to effectuate its general purpose. (b) The general purposes of this chapter are as follows: (1) To establish a more rational system of regulating substances which may pose a danger to the public health; Gabapentin isn’t classified as a controlled substance under federal law in the United States. But it is classified as a controlled substance in some states. Gabapentin is a controlled substance in states like Michigan and Kentucky, while others have mandated reporting rules. Learn about its risk for abuse here. 1. What are the current state regulations for prescription drug dispensing in Rhode Island? The current state regulations for prescription drug dispensing in Rhode Island are overseen by the State Board of Pharmacy and include requirements for controlled substance monitoring, patient identification, prescription labeling, record keeping, and patient counseling. Pharmacists must also adhere to Gabapentin, originally developed to treat epilepsy, has gained popularity as a medication for neuropathic pain and other conditions. However, its increasing use has raised concerns about potential misuse and addiction. As a result, various states have begun to classify gabapentin as a controlled substance. Understanding the legal status of gabapentin across different jurisdictions is crucial
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |