Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
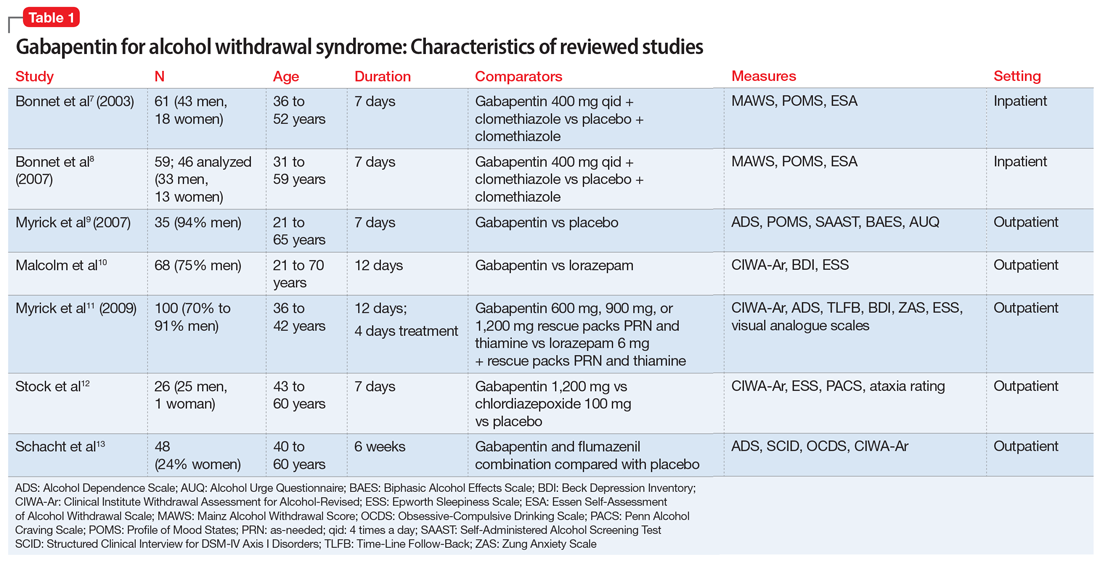
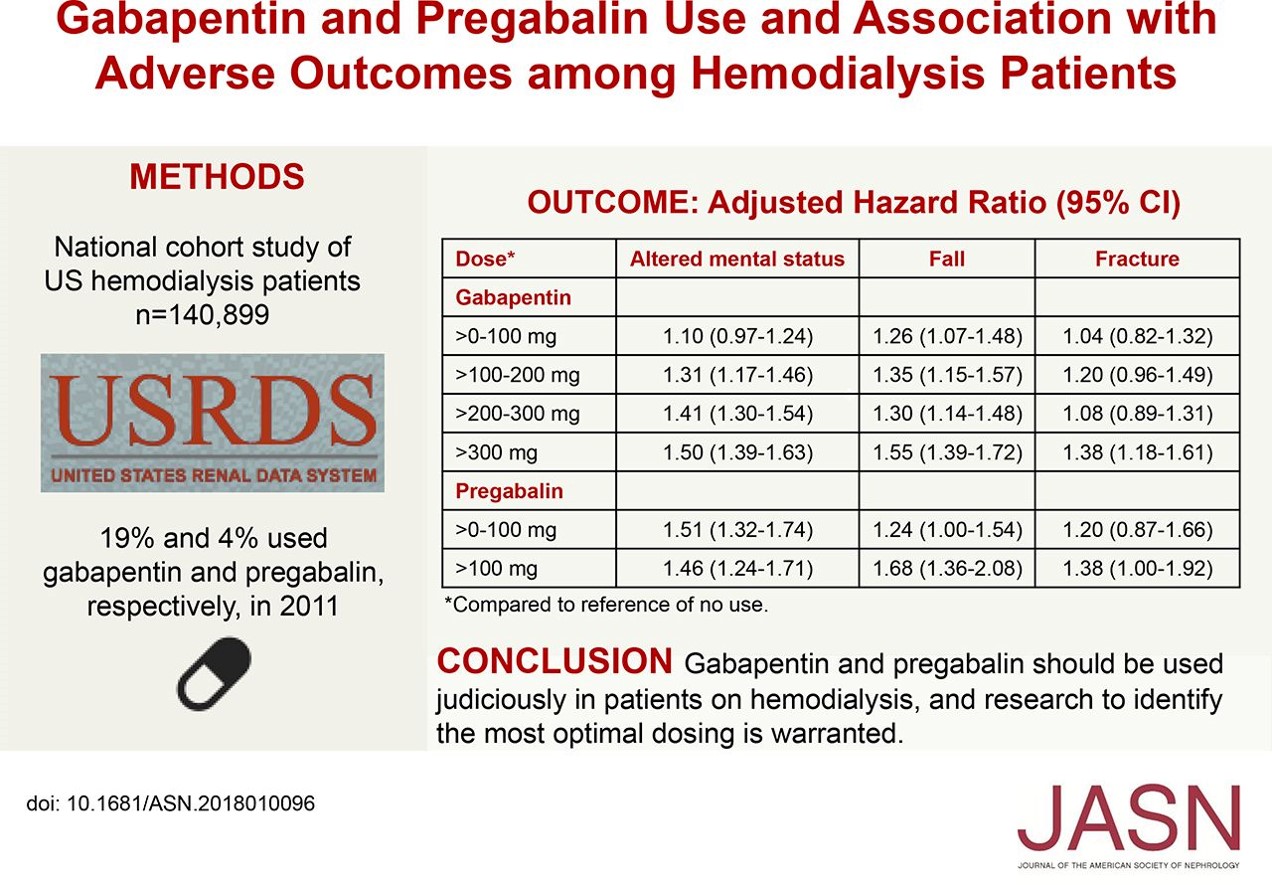
We would like to show you a description here but the site won’t allow us. Learn about gabapentin dosages based on treatment, medical condition, children, and more with GoodRx. The schedule depends on the drug’s potential for abuse or dependency. Schedule V drugs have a lower potential for abuse than all other controlled substances. This means gabapentin has a lower risk of abuse compared to Oxycontin (oxycodone), which is a schedule II opioid medication. Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. This drug is currently marketed in capsule, tablet, and oral solution formulations. In recent years, however, gabapentin has been increasingly encountered by law enforcement, documented in national crime lab Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. Gabapentin reference guide for safe and effective use from the American Society of Health-System Pharmacists (AHFS DI). View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. Detailed Gabapentin dosage information for adults and children. Includes dosages for Restless Legs Syndrome, Epilepsy and Postherpetic Neuralgia; plus renal, liver and dialysis adjustments. Pricing and pack information for Oral tablet, Oral capsule and Oral solution forms of Gabapentin In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Regional Variation Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Gabapentin (standard dose increase) Patient Information The information in this leaflet is to guide your use of gabapentin safely. Further information is available inside the medication packaging. Some medicines used to treat pain symptoms are used for other health reasons. For example some medicines used to treat epilepsy can help to improve nerve pain. Your doctor, nurse or pharmacist will Key takeaways: Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule Gabapentin is a prescription medication approved by the FDA for the treatment of neuropathic pain (postherpetic neuralgia) and seizure disorders. Why is gabapentin controlled in some states? Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation The most common side effect caused by Gabapentin is feeling sleepy, so it is often started at a low dose and built up gradually. Overleaf is a schedule that the Pain Management Service in Wolverhampton recommends. For more information about side effects or contraindications to treatment, you should check the information leaflet inside your medication box, consult your GP, Pharmacist or the Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create A structured gabapentin taper chart helps ease withdrawal and minimize risks, but knowing what works—and what doesn’t—matters just as much. Learn more. Schedule V Controlled Substance in Tennessee July 1, 2018 Effective July 1, 2018, all gabapentin products became Schedule V controlled substances in the state of Tennessee. Gabapentin is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |