Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
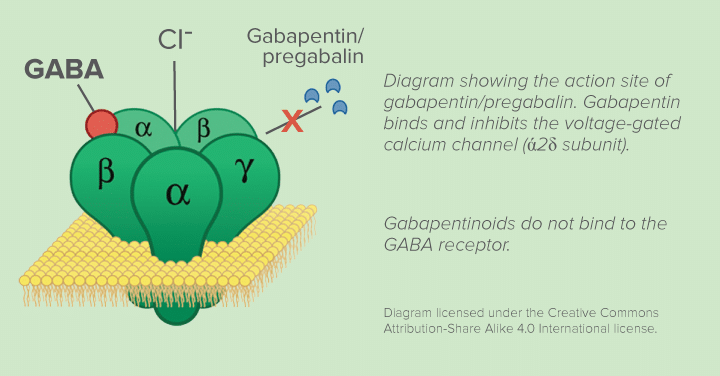 |  |
The objective of this study was to investigate the effect of Lipoderm Cream, VersaBase Gel, and Emollient Cream on the release and permeation of gabapentin formulated for neuropathic pain. Gabapentin of different strengths (1%, 5%, and 10%) was compounded with the bases, diffusion of the drug from thebases, and permeation through artificial skin model studied with Franz diffusionsystem. Steady In this study a variety of topical gabapentin formulations were investigated, including Carbopol ® hydrogels containing various permeation enhancers, and a range of proprietary bases including a compounded Lipoderm ® formulation; furthermore microneedle facilitated delivery was used as a positive control. Gabapentin is mainly prescribed for partial seizures and used off-label for peripheral neuropathic pain.1 Neuropathic pain occurs because of the high sensitivity of calcium channels in the peripheral nerves which results in allodynia and hyperalgesia. Like endogenous neurotransmitter (g-amino-butyric acid [GABA]), topical gabapentin blocks calcium channels and N-methyl-D-aspartate receptors Considering the beneficial anti-neuropathic profile of systemic gabapentin, its central side-effect tendency and the inconsistency of its effectiveness in CIPN, this study investigated the possible efficacy of a gabapentin (10%) topical gel formulation in a refined CIPN rat model of peripheral neuropathic pain. This study provides new data on the potency and stability of topically applied gabapentin compounded without other active ingredients in Lipoderm cream, Versabase gel, and Emollient cream. The present study evaluated the antinociceptive effectiveness of topical gabapentin gel in relation to systemic gabapentin in a well-established cisplatin rat model of CIPN. Objective The aim of this retrospective study was to evaluate the effectiveness and safety of topical gabapentin solution (250 mg/mL) for the management of burning mouth syndrome (BMS). Study design A retrospective chart review was conducted of all patients diagnosed with BMS and managed with gabapentin 250 mg/mL solution (swish and spit) between January 2021 and October 2022. Patient-reported The aim of this retrospective study was to evaluate the effectiveness and safety of topical gabapentin solution (250 mg/mL) for the management of burning mouth syndrome (BMS). A retrospective chart review was conducted of all patients diagnosed with In the mixed-pain group, participants used cream containing ketamine, gabapentin, diclofenac, baclofen, cyclobenzaprine and lidocaine. Cohen cautioned that the new study was somewhat limited in terms of applicability for specific conditions, in part because of the wide variety of medical conditions and pain disorders among the participants. Abstract Topical delivery of gabapentin is desirable to treat peripheral neuropathic pain conditions whilst avoiding systemic side effects. To date, reports of topical gabapentin delivery in vitro have been variable and dependent on the skin model employed, primarily involving rodent and porcine models. In this study a variety of topical gabapentin formulations were investigated, including Topical analgesics are available in sprays, creams, gels, solutions, ointments, and patches [5]. Topical non-steroidal anti-inflammatories (NSAIDs) and lidocaine are often used as single agents. Less common topical analgesics such as gabapentin, ketamine, and baclofen are often used in a compounded topical cream with three or more medications. Another study formulated and characterized gabapentin-encapsulated elastic liposomes and compared their efficiency in transdermal delivery of gabapentin with that of the compounded gabapentin-based Plo lecithin organogel. Conclusions The results of this study suggest that topical gabapentin cream may be effective as a topical agent in the treatment of pruritus associated with PCMA without any significant adverse effects. It is recommended to perform similar studies with a larger sample size and longer duration in both sexes. Topical delivery of gabapentin is desirable to treat peripheral neuropathic pain conditions whilst avoiding systemic side effects. To date, reports of topical gabapentin delivery in vitro have been variable and dependent on the skin model employed, primarily involving rodent and porcine models. In this study a variety of topical gabapentin formulations were investigated, including Carbopol T1 - Topical delivery of gabapentin (Gaba Gel™) for neuropathic pain: A 'proof of concept' study N2 - Focal points • Some patients cannot tolerate oral gabapentin for neuropathic pain due to significant central side effects • Topical gabapentin, an NHS Pharmaceutical unlicensed 'special', has been used as a treatment alternative • Significant reduction in pain scores after treatment The objective of this study was to investigate the effect of Lipoderm Cream, VersaBase Gel, and Emollient Cream on the release and permeation of gabapentin formulated for neuropathic pain. Gabapentin of different strengths (1%, 5%, and 10%) was compounded with the bases, diffusion of the drug from t The topical route also avoids the systemic complications of gabapentin such as somnolence, dizziness, and peripheral edema. This study aimed to perform a narrative synthesis of studies investigating the use of topical gabapentin in the treatment of vulvodynia. The primary outcome was a change in pain score following treatment with topical In this study, various concentrations (1%, 5%, and 10%) of topical gabapentin compounded in Lipoderm were applied at various pre-treatment times (30 minutes, 1 hour, and 4 hours) to investigate what gabapentin concentration and pre-treatment time best attenuates formalin-induced nociceptive behaviors in a rodent model. Significance Systemic gabapentin neuropathic pain management carries side-effects ostensibly preventable by localized therapy. This study validates the effectiveness potential of a topical gabapentin gel against an extensive range of nociceptive stimulus modalities utilizing the chronic constriction injury-induced neuropathic pain model. Adverse effects include somnolence, ataxia, nystagmus, and asthenia, all of which can decrease patient compliance.60,61 Topical gabapentin has been empirically used off-label as a single agent or in combination with amitriptyline and other drugs for neuropathic pain, with a retrospective study suggesting benefit of topical gabapentin for
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |