Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
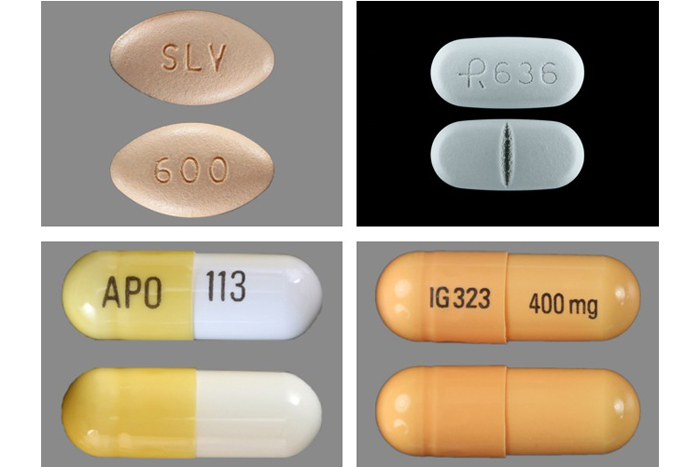
Gabapentin is an anti-epileptic drug, also called an anticonvulsant. It is used to treat some types of seizures and nerve pain caused by shingles. Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication used to treat partial seizures, neuropathic pain, hot flashes, and restless legs syndrome. [2][3] It is recommended as one of a number of first-line medications for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central neuropathic pain. [8] About 15% of Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gabapentin has the potential of an anticonvulsive medication and can be used as an adjunct to more Gabapentin (Neurontin®, Gralise®) is FDA-approved for use in adults with postherpetic neuralgia (PHN). Neurontin® is also approved as adjunctive therapy for managing partial seizures with or without secondary generalization in patients with epilepsy.1-4 Gabapentin enacarbil (Horizant®) is FDA approval history for Horizant (gabapentin enacarbil) used to treat Restless Legs Syndrome, Postherpetic Neuralgia. Supplied by Azurity Pharmaceuticals, Inc. Gabapentin, a GABA receptor agonist, was first studied as an antiepileptic drug in humans in 1987 (07). It was launched in the United Kingdom in 1993 and approved in the United States as add-on therapy for intractable partial seizures in adults. It is also approved for the treatment of postherpetic neuralgia. FDA approval history for Gralise (gabapentin) used to treat Postherpetic Neuralgia. Supplied by Depomed, Inc. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 One such drug, gabapentin (Neurontin), received approval by the U.S. Food and Drug Administration (FDA) in 1993 as an adjunct medicine for partial seizures and additional FDA approval in 2002 for Note: If you need help accessing information in different file formats, see . Language Assistance Available: | | | | | | | | | | | | | | | Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] It is moderately effective: about 30–40% of those given Generic Gralise Availability Last updated on Jun 11, 2025. Gralise is a brand name of gabapentin, approved by the FDA in the following formulation (s): GRALISE (gabapentin - tablet;oral) Manufacturer: ALMATICA Approval date: January 28, 2011 Strength (s): 300MG [RLD] [AB2], 600MG [RLD] [AB2] Manufacturer: ALMATICA Approval date: April 18, 2023 Strength (s): 450MG [RLD], 750MG [RLD], 900MG [RLD Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia. Gabapentin (Neurontin, Gralise, Horizant) was first approved by the US Food and Drug Administration (FDA) in 1993 for the treatment of epilepsy, and in 2004 was approved for the treatment of post-herpetic neurological pain. Gabapentin was approved by the US Food and Drug Administration in 1993 to control partial seizures in epilepsy patients older than 12. Gabapentin (Neurontin) FDA-approved indications and off-label uses; gabapentin withdrawal and abuse potential; mechanism of action; how long it takes for gabapentin to start working. Gabapentin gained FDA approval in 1993 under the brand name Neurontin as an adjunctive therapy in the treatment of partial onset seizures, and subsequently for the treatment of postherpetic neuralgia in adults in 2002. It became available as a generic in 2004.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |