Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
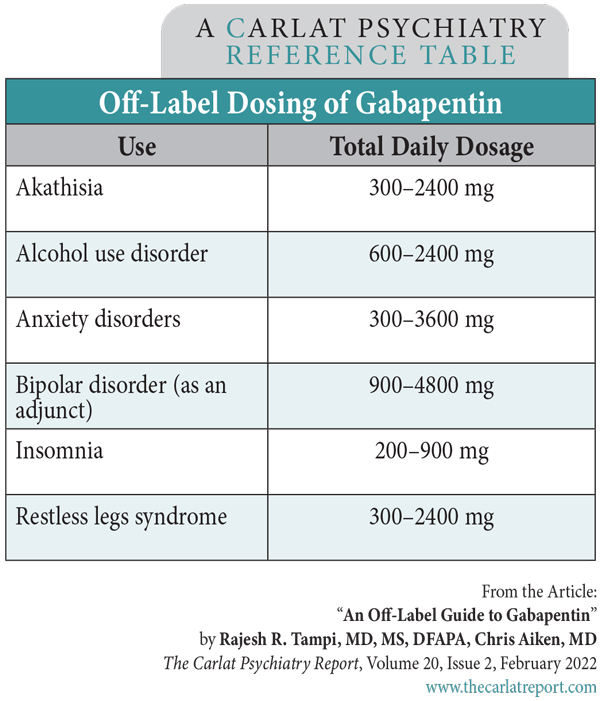
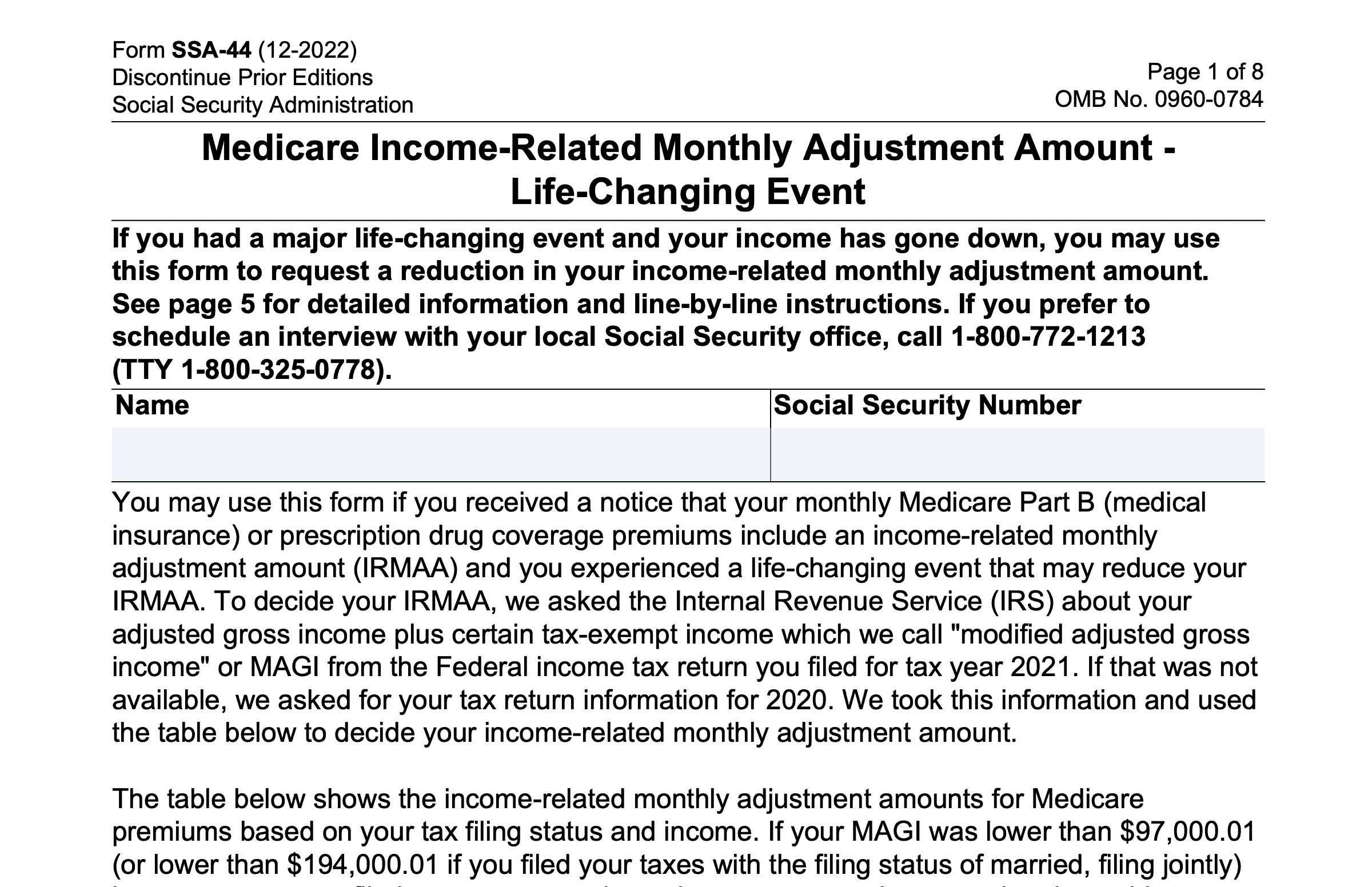
Web a gabapentin lawsuit was filed in 2022, alleging that the manufacturers of gabapentin failed to warn patients about. Web the purpose of this proof of claim form and release is to ensure that you are able to participate in the distribution of the. Web there are different types of lawsuits related to gabapentin, including individual lawsuits, class action lawsuits,. Gabapentin Lawsuit Form - Web in addition, upon the settlement becoming final, plaintiffs and each class member, on behalf of themselves and all other releasors, will expressly waive, release. Dangerously overprescribed gabapentin should be classified as a controlled substance. Evidence shows gabapentin is often misused and abused,. Gabapentin capsules are solid dоsage forms that contain the Neurontin Lawsuit | 2025 Latest Updates The prescription pain reliever Neurontin (generic gabapentin) has recently been linked to an increased risk for Stevens-Johnson syndrome (SJS), a severe skin disorder in which the top layer of the skin dies, followed by a painful rash that spreads and blisters. The purpose of this Proof of Claim Form and Release is to ensure that you are able to participate in the distribution of the Settlement Fund, net of attorneys’ fees, expenses, incentive awards, and claims administration costs (the “Net Settlement Fund”) if the Settlement is finally approved by the Court. Based on Pfizer’s and generic gabapentin manufacturers’ electronic sales data Los Angeles, CA: A $325 million preliminary settlement has been reached by Pfizer Inc and Warner-Lambert Co. LLC and plaintiffs who filed a consumer fraud class action lawsuit over the marketing How To Join Gabapentin Lawsuit? In this insightful video, we delve into the process of joining a Gabapentin lawsuit. If you've been prescribed Gabapentin and believe you've suffered harm from its The plaintiffs in the Neurontin class action lawsuit alleged that Pfizer delayed competition from generic producers of the drug, gabapentin anhydrous, which Pfizer sold under the name Neurotin, by exercising a monopoly over the medicine. View the Neurontin Antitrust Litigation Claim Form Instructions in our collection of PDFs. Sign, print, and download this PDF at PrintFriendly. UNITED STATES DISTRICT COURT FOR THE DISTRICT OF NEW JERSEY SUMMARY NOTICE OF PROPOSED CLASS ACTION SETTLEMENT, MOTION FOR ATTORNEYS’ FEES, AND HEARING REGARDING SETTLEMENT TO: ALL PERSONS OR ENTITIES WHO HAVE PURCHASED NEURONTIN DIRECTLY FROM PFIZER, INC. AND WARNER-LAMBERT AT ANY TIME DURING THE PERIOD OF DECEMBER 11, 2002, THROUGH AUGUST 31, 2008 AND WHO HAVE ALSO PURCHASED GENERIC A proposed settlement has been reached in a class action lawsuit against Pfizer Inc. and Warner-Lambert Company LLC regarding the marketing of the prescription drug Neurontin. The class action settlement provides cash awards to Third-Party Payors (TPP) of Neurontin, including all insurance companies, health benefit providers, and entities that provide prescription drug benefits and/or pharmacy The manufacturers of the drug Neurontin reached a $325 million class action lawsuit settlement Wednesday over allegations they fraudulently marketed the prescription drug, which is used to treat seizures, restless leg syndrome, and pain caused by shingles. If approved, third-party payers of the drug will be eligible to claim a cash award from the Neurontin class action settlement, announced Dr David Franklin, a former employee of the Warner- Lambert Pharmaceutical Company, has filed a lawsuit against the company, alleging that its sales representatives encouraged doctors to prescribe gabapentin (Neurontin) for unapproved uses. Gabapentin was approved by the Food and Drug Administration in 1994 for the treatment of epilepsy, including elementary partial seizures and complex Legally Reviewed Fact-Checked What Is Neurontin? Gabapentin, an anti-seizure drug marketed by Pfizer Laboratories in the United States under the trademark name Neurontin, has been the subject of numerous claims of malfeasance in gabapentin side effects lawsuits, with the Pfizer company intentionally and knowingly marketing the medication for uses not approved by the FDA (Food and Drug If you have been prescribed Neurontin, bought and/or used the medication for a use other than one which is FDA-approved, and suffered any of the above side effects, please please fill out the form Explore the potential for seeking compensation in gabapentin-related memory loss cases, including legal grounds, evidence, and filing deadlines. · The purpose of this notice is to alert you to a proposed settlement of a Class Action Lawsuit (the “Lawsuit”) brought by Direct Purchasers of Neurontin against Pfizer Inc. and Warner-Lambert Company LLC (collectively “Pfizer” or “Defendants”). The Lawsuit asserts that Pfizer violated antitrust laws relating to the sale of its prescription drug Neurontin. An $85 million class action has been reached with Sun Pharmaceutical Industries and Taro Pharmaceuticals. The class action lawsuit claims that certain generic drug manufacturers broke antitrust laws, leading to artificially higher prices for common generic drugs such as amphetamine, amoxicillin, gabapentin, baclofen, and others. Gabapentin, commonly sold under the brand name Neurontin, is a prescription drug used to treat seizures, restless leg syndrome, and pain caused by shingles. While it has been effective for some patients, there have been several cases of adverse effects and potential health concerns associated with its use. In recent years, the manufacturers of Gabapentin have faced legal challenges as a result The gabapentin class action lawsuit was filed in 2022 against three pharmaceutical companies – Teva, Pfizer, and Greenstone – accusing them of misrepresenting the drug’s risks and overstating its benefits.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |